Case Report - Journal of Interdisciplinary Histopathology (2022)
An Unsuspected Diagnosis for a Colonic Necrotic Mass
A.A. Arteta1, O.M. Ardila Suarez2 and Mario A. Caviedes Cleves3*2Grupo de Investigaciones en Patología, Universidad de Antioquia, Medellín, Colombia
3Department of Pathology, University of Antioquia, Medellín, Colombia
Mario A. Caviedes Cleves, Department of Pathology, University of Antioquia, Medellín, Colombia, Email: mario.caviedes@udea.edu.co
Received: 07-Jul-2022, Manuscript No. EJMJIH-22-68803; Editor assigned: 12-Jul-2022, Pre QC No. EJMJIH-22-68803 (PQ); Reviewed: 26-Jul-2022, QC No. EJMJIH-22-68803; Revised: 02-Aug-2022, Manuscript No. EJMJIH-22-68803 (R); Published: 09-Aug-2022
Abstract
Schwannomas are benign peripheral nerve sheath tumors derived from Schwann cells which rarely arise in the soft tissues of the gastrointestinal tract. We present a case of a gastrointestinal schwannoma in a previously healthy 50-years-old female, with no family history of congenital diseases. The main symptom of the patients was lower gastrointestinal bleeding. A CT scan and colonoscopy described a 4 cm ulcerated polypoid mass in the descending colon. The microscopic morphology of the lesion and the immunohistochemical profile were consistent with a schwannoma. The patient had a favorable evolution after surgical resection.
Keywords
Schwannoma; Colon; Mesenchymal tumors; gastrointestinal tract; Polypoid mass
Introduction
Lower gastrointestinal bleeding is an ominous symptom associated with a wide range of etiologies among them neoplasms [1]. Most of the neoplasms arising in the gastrointestinal tract are from the epithelium or mesenchyme, with primary hematologic neoplasms representing a very low proportion of total tumors [2]. Epithelial and mesenchymal tumors can have a polypoid endoscopic configuration, with histopathological tissue analysis being necessary for distinction and definitive diagnosis. Neoplasms deriving from the epithelium are most commonly adenomas and adenocarcinomas while those deriving from the mesenchyme are primarily Gastrointestinal Stromal Tumors (GIST) [3]. Furthermore, while tumors of a neural origin in the gastrointestinal tract a rare, a wide range of these tumors have been described [4-6]. We present a case of a symptomatic gastrointestinal schwannoma presenting as an ulcerated colonic sessile mass.
Case Presentation
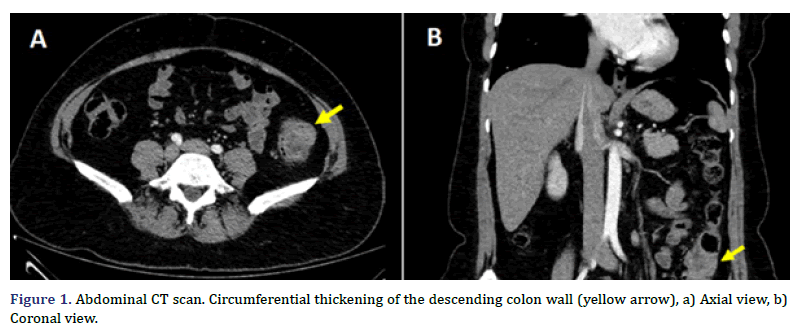
A previous healthy 50 years-old female arrived at the emergency department due to severe diffuse abdominal pain, and bloody diarrhea. The patient had been suffering from similar, mild symptoms for about three months and did not seek previous medical assistance before, for fear of being infected with COVID-19. Physical examination was unremarkable except for a slight, diffuse pain during abdominal palpation, without tenderness. She had a surgical history of cesarean section and surgical sterilization, and there was not prior family medical history of congenital diseases. An abdominal CT scan revealed a circumferential thickening on the descending colon with trans-mural involvement, and an increased density of the peripheral fat, with a probable neoplastic origin (Figures 1A and 1B), associated with mesenteric adenopathy.
The characteristics of the remaining abdominal organs were unremarkable. Upper endoscopy showed a follicular gastritis, positive for Helicobacter pylori in the histopathological analysis, associated with a chronic inflammatory infiltrate with lymphoid follicles formation- most of them with germinal centers - and moderate acute infiltrate.
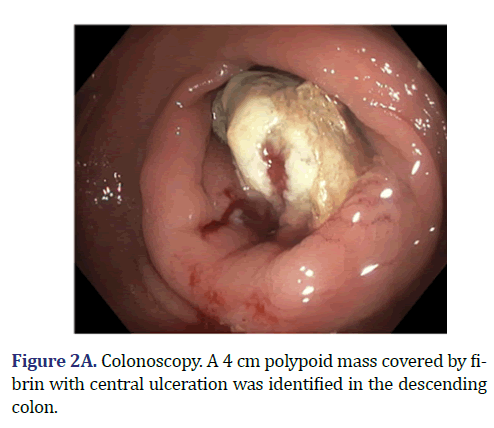
Colonoscopy demonstrated a 4 cm sessile mass located in the descending colon, 40 cm from the anal verge, covered with fibrin and necrotic tissue (Figure 2A). The lesion occupied around 80% of the lumen, and multiple biopsies were obtained. The histopathological analysis of the biopsies reported necrotic tissue, fibrin and cellular debris without neoplastic features.
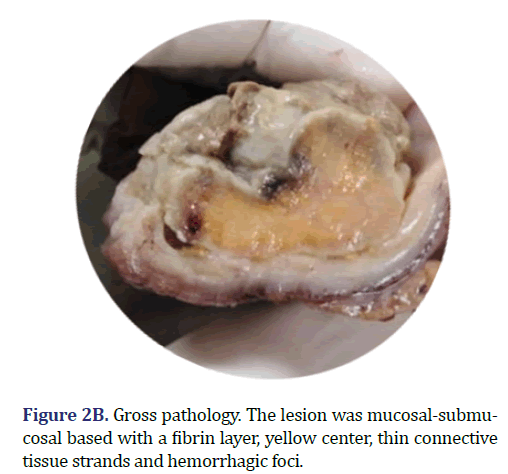
The patient underwent laparoscopic left hemicolectomy. A 15 cm long colon segment arrived at the pathology laboratory, upon the mucosal surface of which a 4 cm sessile polypoid mass, cover in fibrin was identified (Figure 2B). The lesion was mucosal-submucosal based; the center of the lesion was yellow with hemorrhagic foci, without thickening of the colonic wall. Multiple lymph nodes were extracted from the mesenteric fat.
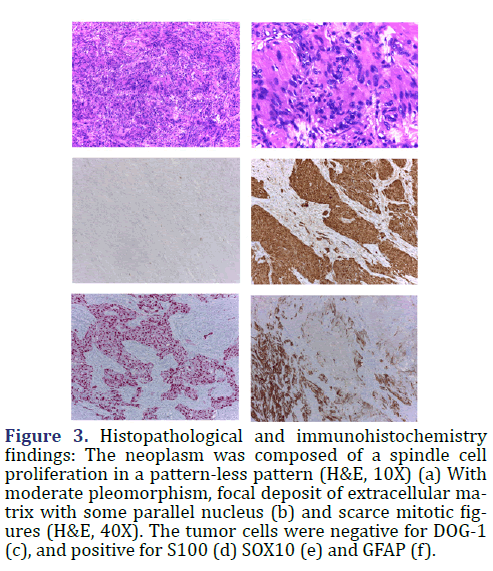
In the histopathological analysis, the polypoid mass was ulcerated at the surface with a cap of granulation tissue, fibrin, cellular debris and acute inflammatory infiltrate. The center of the lesion was composed of well-circumscribed spindle cell proliferation. The spindle tumor cells had tapered nucleus, uniform chromatin with a single nucleolus, and a fascicular and sheet-like growth pattern. There were moderate pleomorphisms, less than 5 mitotic figures in 50 high power fields, and no variable cellular Antoni A and Antoni B areas or Verocay bodies. The stroma of the tumor was collagenous, hypocellular, with lymphocytic inflammatory infiltrate and small arterial vessels with hyalinized walls. The immunohistochemical profile (Figure 3) shows diffuse nuclear and cytoplasmatic S100 expression and positivity for SOX10 and GFAP (Glial Fibrillary Acid Protein). The tumor cells were negative for KIT, DOG1, SMA, Desmin, Caldesmon, HMB45, Melan-A, ALK-1, CD34, CD31, EMA, Chromogranin, Synaptophysin, CD56, TLE-1, EMA and Cytokeratins. With the morphological and the immunohistochemical profile, the colonic polypoid mass was diagnosed as a Schwannoma.
Figure 3. Histopathological and immunohistochemistry findings: The neoplasm was composed of a spindle cell proliferation in a pattern-less pattern (H&E, 10X) (a) With moderate pleomorphism, focal deposit of extracellular matrix with some parallel nucleus (b) and scarce mitotic figures (H&E, 40X). The tumor cells were negative for DOG-1 (c), and positive for S100 (d) SOX10 (e) and GFAP (f).
Discussion
Schwannomas are benign peripheral nerve sheath tumors derived from Schwann cells which rarely arise in the soft tissues of the gastrointestinal tract. Schwann cells, discovered by Theodore Ambrose Hubert Schwann almost two centuries ago, are specialized cells dedicated to axon myelinization and nerve trauma repair [7]. Schwannomas can arise sporadically or in the context of a few congenital conditions associated with multiple schwannomas, such as familial schwannomatosis, Carney complex syndrome and neurofibromatosis type 2 [8]. The tumorigenesis of these soft tissue tumors in type 2 neurofibromatosis, is associated with the loss of the suppressor gene NF2 function, located on chromosome 22q12, which encodes Merlin (moezin-ezrin- radixin like protein, also known as schwannomina) [9]. Merlin acts as a scaffold protein indirectly linking F-actin, modulating cell signals that promote cell survival and proliferation through, among others, tyrosine kinase receptors, cell adhesion, small GTPases, mammalian Target of Rapamycin (mTOR) and PI3K/Akt [10].
Within the gastrointestinal tract, schwannomas are usually found in the gastric corpus and in the right colon. There is a wide age range with peak incidence in the seventh decade and a female predominance for gastric schwannomas [11]. Colonic schwannomas have no gender predominance, they are usually located on the cecum, and the macroscopic features vary from polypoid masses to bulging over the serosal surface. Schwann cells are associated with the Auerbach´s and Meissner´s plexus [12]. We therefore, hypothesized that if the tumorigenic Schwann cell is in the submucosal plexus the tumor configuration could be polypoid or intraluminal. Additionally, if the tumorigenic Schwann cell is in the myenteric plexus the tumor configuration could be non-luminal.
The presentation of symptoms depends on the location and macroscopic configuration of the tumor. Clinical presentation of schwannoma as a polypoid intraluminal mass is rare, and this macroscopic configuration tends to produce lower gastrointestinal bleeding, or obstruction symptoms [13]. Other symptoms such as vague abdominal pain or even the clinical presentation of an incidentally gastrointestinal mass detected by radiological imaging have also been reported in gastrointestinal schwannoma cases [14, 15]. Presurgical diagnosis is the exception and not the rule, and just a few cases have been diagnosed in pre-surgical specimens. The rarity of the tumor in the gastrointestinal tract, the depth of the tissue obtained through the endoscopic procedure, submucosal location and confounding biopsy factors such as ulceration and severe inflammatory infiltrate hinder a precise diagnosis in biopsy specimens. In the diagnostic work-up of a mesenchymal mucosal-submucosal gastrointestinal lesion, there is a wide differential diagnosis [16-18].
Conclusion
There were not histopathological criteria for malignancy, Gastro Intestinal Stromal Tumor (GIST), was ruled out with the negativity of KIT, DOG1 and CD34, as well as muscular tumors due to negativity of Desmin and SMA. The strong nuclear and cytoplasmic positivity for S100 protein narrowed the differential diagnosis in this case. The morphology was not typical for Clear Cell Sarcoma like Gastrointestinal Tumor; usually these kinds of tumors are negative for GFAP, and positive for neuroendocrine markers. The benign morphology, the combined positivity for S100, SOX10 and GFAP after ruling out other mesenchymal tumors, favored the diagnosis of schwannoma.
References
- Bai Y, Peng J, Gao J, Zou D-W, Li Z-S. Epidemiology of lower gastrointestinal bleeding in China: Single-center series and systematic analysis of Chinese literature with 53,951 patients. J Gastroenterol Hepatol 2011;26:678–82.
[Crossref] [Google scholar] [Pub Med]
- Gustafsson BI, Siddique L, Chan A, Dong M, Drozdov I, Kidd M, et al. Uncommon cancers of the small intestine, appendix and colon: An analysis of SEER 1973-2004, and current diagnosis and therapy. Int J Oncol 2008;33:1121–31.
[Crossref] [Google scholar] [PubMed]
- Charville GW, Longacre TA. Surgical Pathology of Gastrointestinal Stromal Tumors: Practical Implications of Morphologic and Molecular Heterogeneity for Precision Medicine. Adv Anat Pathol 2017;24:336–53.
[Crossref] [Google scholar] [PubMed]
- Chintanaboina J, Clarke K. Case of colonic mucosal Schwann cell hamartoma and review of literature on unusual colonic polyps. BMJ Case Rep. 2018; bcr2018224931.
[Crossref] [Google scholar] [PubMed]
- van Wyk AC, van Zyl H, Rigby J. Colonic perineurioma (benign fibroblastic polyp): Case report and review of the literature. Diagn Pathol 2018;13:16.
[Crossref] [Google scholar] [PubMed]
- Rawal G, Zaheer S, Ahluwalia C, Dhawan I. Malignant peripheral nerve sheath tumor of the transverse colon with peritoneal metastasis: A case report. J Med Case Reports 2019;13:15.
[Crossref] [Google scholar] [PubMed]
- Sonig A, Gandhi V, Nanda A. From the cell of Schwann to schwannoma--a century’s fruition. World Neurosurg 2014;82:906–11.
[Crossref] [Google scholar] [PubMed]
- Carroll SL, Ratner N. How does the Schwann cell lineage form tumors in NF1?. Glia 2008;56:1590–605.
[Crossref] [Google scholar] [PubMed]
- Petrilli AM, Fernández-Valle C. Role of Merlin/NF2 inactivation in tumor biology. Oncogene 2016;35:537–48.
[Crossref] [Google scholar] [PubMed]
- Stamenkovic I, Yu Q. Merlin, a “magic” linker between extracellular cues and intracellular signaling pathways that regulate cell motility, proliferation, and survival. Curr Protein Pept Sci 2010;11:471–84.
[Crossref] [Google scholar] [PubMed]
- Bohlok A, El Khoury M, Bormans A, Galdon MG, Vouche M, El Nakadi I, et al. Schwannoma of the colon and rectum: A systematic literature review. World J Surg Oncol 2018;16:125.
[Crossref] [Google scholar] [PubMed]
- Tsunoda C, Kato H, Sakamoto T, Yamada R, Mitsumaru A, Yokomizo H, et al. A Case of Benign Schwannoma of the Transverse Colon with Granulation Tissue. Case Rep Gastroenterol 2009;3:116–20.
[Crossref] [Google scholar] [PubMed]
- Miettinen M, Shekitka KM, Sobin LH. Schwannomas in the colon and rectum: A clinicopathologic and immunohistochemical study of 20 cases. Am J Surg Pathol 2001;25:846–55.
[Crossref] [Google scholar] [PubMed]
- Goh BKP, Chow PKH, Kesavan S, Yap W-M, Ong H-S, Song I-C, et al. Intraabdominal schwannomas: A single institution experience. J Gastrointest Surg 2008;12:756–60.
[Crossref] [Google scholar] [PubMed]
- Uhr A, Singh AP, Munoz J, Aka A, Sion M, Jiang W, et al. Colonic Schwannoma: A Case Study and Literature Review of a Rare Entity and Diagnostic Dilemma. Am Surg 2016;82:1183–6.
[Crossref] [Google scholar] [PubMed]
- Hirota S. Differential diagnosis of gastrointestinal stromal tumor by histopathology and immunohistochemistry. Transl Gastroenterol Hepatol 2018;3:27.
[Crossref] [Google scholar] [PubMed]
- Doyle LA, Hornick JL. Mesenchymal Tumors of the Gastrointestinal Tract Other than GIST. Surg Pathol Clin 2013;6:425–73.
[Crossref] [Google scholar] [PubMed]
- Wang J, Thway K. Clear cell sarcoma-like tumor of the gastrointestinal tract: An evolving entity. Arch Pathol Lab Med 2015;139:407–12.
[Crossref] [Google scholar] [PubMed]
Copyright: © 2022 The Authors. This is an open access article under the terms of the Creative Commons Attribution NonCommercial ShareAlike 4.0 (https://creativecommons.org/licenses/by-nc-sa/4.0/). This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.