Research Article - Journal of Interdisciplinary Histopathology (2022)
Apatite Rheumatism and Chondrocalcinosis are Different Stages of the Same Metabolic Disorder: A Clinicopathologic Study of 21 Patients with Clinically Diagnosed Apatite Rheumatism or Chondrocalcinosis
Miklós Bély1* and Ágnes Apáthy22Department of Rheumatology, St. Margaret Clinic,, Budapest, Hungary
Miklós Bély, Department of Pathology, Hospital of the Order of the Brothers of Saint John of God in Budapest, Budapest, Hungary, Email: dr.bely.miklos@gmail.hu
Received: 13-Jul-2022, Manuscript No. EJMJIH-22-69181; Editor assigned: 25-Jul-2022, Pre QC No. EJMJIH-22-69181 (PQ); Reviewed: 08-Aug-2022, QC No. EJMJIH-22-69181; Revised: 16-Aug-2022, Manuscript No. EJMJIH-22-69181 (R); Published: 23-Aug-2022
Abstract
Introduction: Apatite rheumatism and chondrocalcinosis are considered to be different metabolic diseases and distinct clinical entities, although the clinical symptoms are the same, the most commonly affected joints overlap, and the currently available treatment is similar. Original descriptions, interdisciplinary studies and excellent clinicopathology manuals also support this view, since the Hydroxy Apatite (HA) crystals are virtually not existing in conventionally stained tissue sections, while the Calcium Pyrophosphate Dehydrate (CPPD) crystals may be retained. The introduction of the non-staining technique by Bély and Apáthy opened a new era in the diagnosis of crystal induced diseases, allowing the analysis of cholesterol, HA, CPPD, Mono Sodium Urate monohydrate (MSU) crystals, and others in traditionally fixed, paraffinembedded, non-stained tissue sections. The aim of this study was to analyze HA and CPPD deposits in 5 patients with clinically diagnosed apatite rheumatism, and in 16 patients with clinically diagnosed chondrocalcinosis.
Materials and Methods: Twenty-six joints of 21 patients with crystal induced metabolic diseases were operated; a total of 57 tissue samples were analyzed.
Results: Different amounts of HA and CPPD crystals were detected in tissue sections of all 21 patients by non-staining technique. Crystal induced metabolic diseases started with deposition of HA crystals, followed by CPPD, and amorphous calcium phosphate and/or carbonate deposition.
Discussion and Conclusions: The dominance of HA and/or CPPD crystals is much more characteristic of clinically diagnosed apatite rheumatism or chondrocalcinosis, in comparison to the similar clinical symptoms or affected joints. Crystal-induced metabolic diseases are progressive cumulative diseases, and apatite rheumatism and chondrocalcinosis may be considered different stages of the same metabolic disorder, rather than two separate entities. According to the “most common is the first” hypothesis, the metabolic disease caused by HA and CPPD crystals, begins in the synovial membrane of the knee joint.
Keywords
Apatite rheumatism; Chondrocalcinosis; Calcium hydroxyapatite; Calcium pyrophosphate dehydrates; Conventional stains and histochemical reactions; Non-staining technique
INTRODUCTION
Arthropathy induced by hydroxyapatite (Ca5(PO4)3(OH)) (HA) crystals (apatite rheumatism, hydroxyapatite arthritis, calcifying tenosynovitis, Milwaukee syndrome, frozen shoulder, calcific tendinitis, calcific bursitis, calcific periarthritis, periarthritis calcarea) and arthropathy induced by Calcium PyroPhosphate Dihydrate (Ca2P2O7.2H2O) (CPPD) crystals (chondrocalcinosis, polyarticular (familial) chondrocalcinosis, pseudogout, pyrophosphate arthropathy) are regarded as different metabolic diseases, and distinct clinical entities [1–11], although the clinical symptoms are the same, the most commonly affected joints overlap, and the currently available treatment is similar [12–16]. The solubility of HA and CPPD crystals is different in conventional fixatives (aqueous formaldehyde solution), in ethyl alcohol, acetone, xylene and in aqueous solutions of dyes.
In fixed tissues embedded in paraffin and stained with traditional dyes the more soluble small and weak birefringent HA crystals are virtually non-existent (there is no detectable birefringence) [7-11], while the less soluble larger and strongly birefringent CPPD crystals may occasionally be preserved and demonstrated under polarized light [6–8]. The HA or CPPD crystals provoke acute inflammatory reactions, with polymorphonuclear neutrophilic and eosinophilic leucocytes, monocytes, and multinucleated giant cells.
Apatite Rheumatisms (AR) and CHondro-Calcinosis (Ch- C) may be accompanied by more or less abundant amorphous calcium phosphate (CaPO4) or calcium carbonate (CaCO3) deposition, which may mask the only, exceptionally preserved HA or occasionally retained CPPD crystals in traditionally stained tissue samples.
The amorphous calcium phosphate or carbonate deposits (with or without demonstrable HA or CPPD crystal contents) are characterized by nearly complete absence of an inflammatory reaction, surrounded by fibrotic soft tissue and dense collagen fibers.
In conventionally fixed and in paraffin embedded but unstained tissue sections surprisingly many crystals can remain, indicating that most of the crystals dissolve during staining. The “non-staining” techniques by Bély and Apáthy [17] is a very sensitive method, and suitable to identify cholesterol (C27H46O), calcium hydroxyapatite (Ca5(PO4)3(OH)) (HA), calcium pyrophosphate dehydrate (Ca2P2O72H2O) (CPPD) crystals, and appropriate for detection of monosodium urate monohydrate (NaC5H- 3N4O3•H2O) (Monosodium Salt of Uric acid (C5H4N4O3) – MSU) crystals, etc. in formalin fixed, paraffin embedded, unstained tissue sections viewed with polarized light [17–30].
The aim of this study was:
1. To analyse the dominant crystal depositions in patients with clinically diagnosed apatite rheumatism and chondrocalcinosis by non-staining technique according to Bély and Apáthy,
2. To compare the conventional, HE stained histological sections with non-stained ones, and
3. To determine the possible deposition order (sequence of deposition) of HA (Ca5 (PO4)3(OH)), CPPD (Ca2P2O7.2H2O) crystals, and of amorphous calcium phosphate (CaPO4) or calcium carbonate (CaCO3) in tissues.
Materials and Methods
Twenty-six (26) joints (knee n=14, hip n=5, shoulder n=4, wrist n=2, elbow n=1) causing complaints were operated in 21 patients with crystal induced metabolic diseases.
In total 57 tissue samples were analysed; surgical specimens (n=13) and synovial needle biopsies (n=4) of 5 patients with clinically diagnosed apatite rheumatism (AR), and surgical specimens (n=40) of 16 patients with clinically diagnosed CHondro-Calcinosis (Ch-C) were studied. The tissue blocks were fixed in an 8% aqueous solution of formaldehyde (CH2 (OH)2) at pH 7.6 for >24 hours at room temperature (20oC) and embedded in paraffin. Serial tissue sections (5 microns) of apatite rheumatism and chondrocalcinosis were stained with HE [31], Alizarin red S (staining specific for calcium) [32,33], and von Kossa’s reaction (specific for phosphate (CaPO4) or carbonate (CaCO3) [32,34] and were compared with unstained ones [29].
Using Bély and Apáthy’s “non-staining” technique [17– 30], the fixation of tissue blocks and embedding in paraffin were the same as with the standard staining’s and reactions. Tissue sections were deparaffinised, mounted and cover slipped with Canada balsam without staining procedures (description of method see Appendix). The cellular composition of inflammatory infiltration (in selected cases) was identified by immunohistochemical techniques using the streptavidin-biotin-complex/horseradish peroxidase method [35].
Standard and unstained tissue sections were examined with the light microscope (Olympus BX51) and under polarized light, respectively. Prevalence of deposited crystals in tissue sections stained by conventional HE and in nonstained ones was compared by Pearson’s chi-squared (χ2) test [36]. Appendix–Bély and Apáthy’s “non-staining” technique [17–30].
1. Tissue blocks of surgically removed specimens were fixed in 8% neutral buffered formalin (at pH 7.6 for >24 hours at 20 oC room temperature).
2. Tissue blocks were dehydrated in ethyl alcohol, and were embedded in paraffin using acetone as well as xylene– 5 μm sections was cut.
3. Prolonged deparaffinization (3-5 days) in a thermostat at 56°C (daily changing xylene)
4. Chloroform–methanol I. (1:1) solution for 1 hour
5. Chloroform–methanol II. (1:1) solution for 1 hour or overnight
6. Dehydration in ethyl alcohol (two changes of 96% alcohol I-II. 30-30 min.), and using terpene xylene, as well as xylene, mount in Canada balsam, cover slip.
Results
1. HA and CPPD crystal deposits in surgical specimens (n=57) of 21 patients with clinically diagnosed apatite rheumatism (n=5) and chondrocalcinosis (n=16)
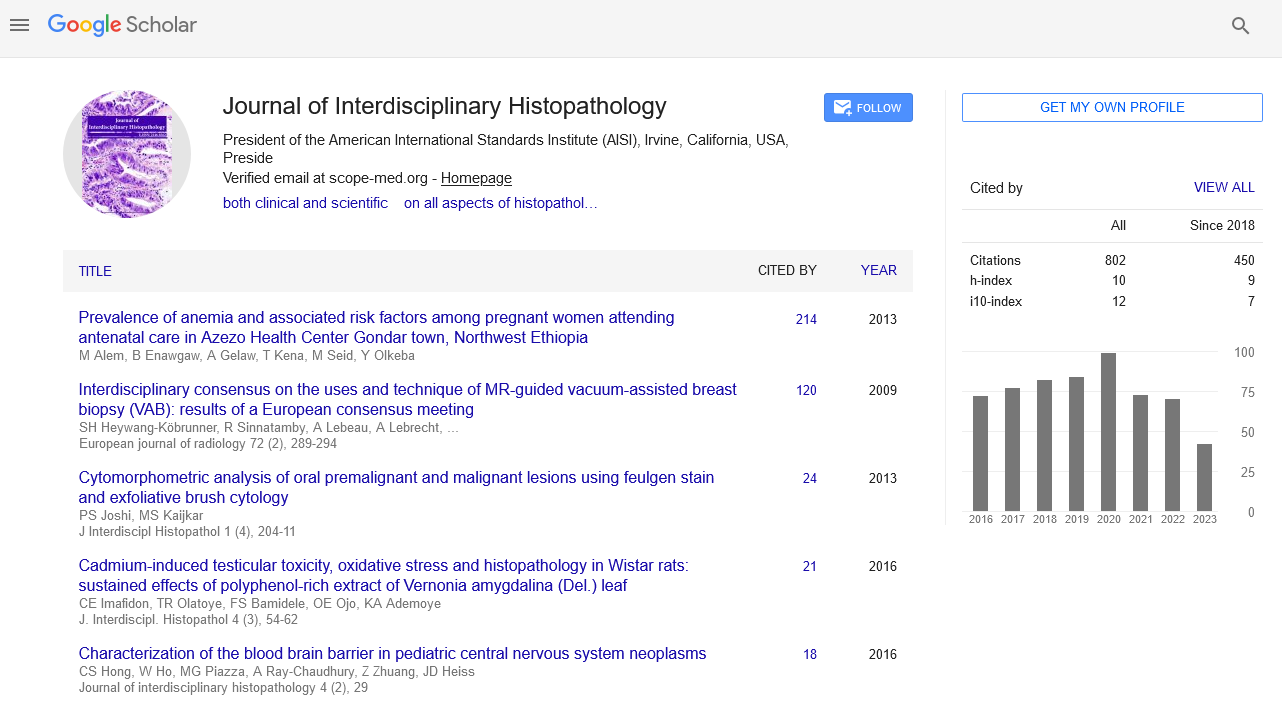
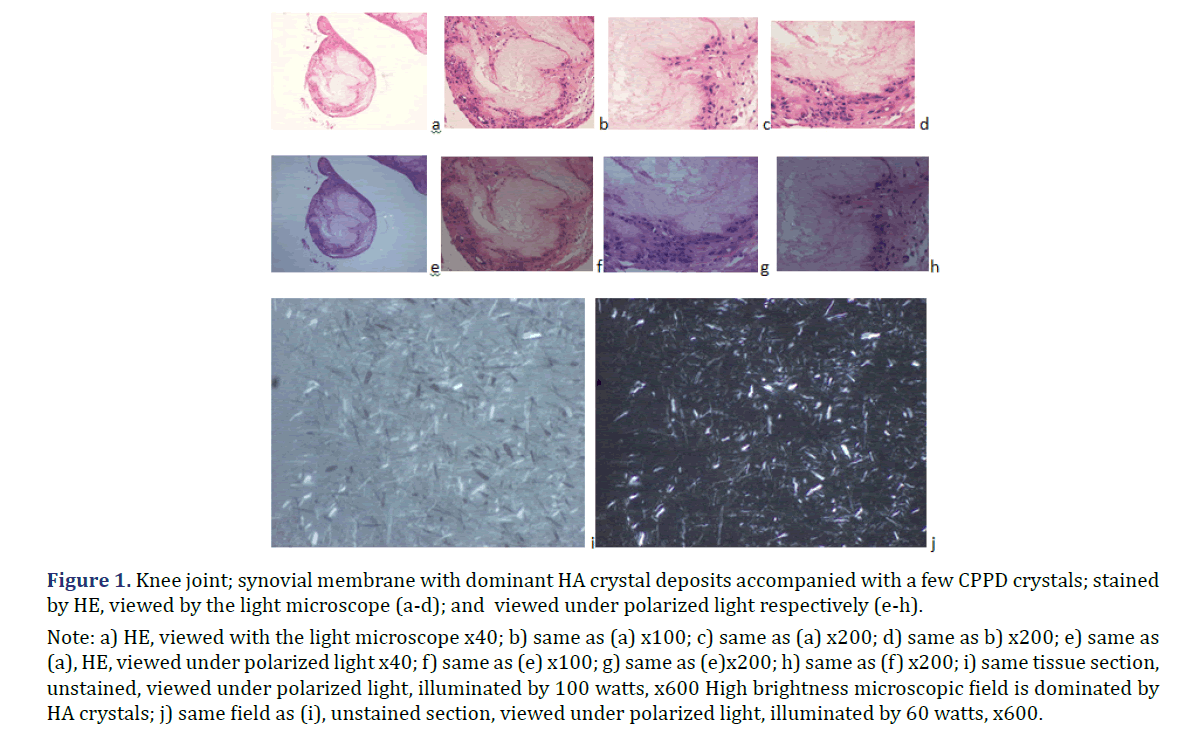
In all 21 patients with clinical diagnosis of apatite rheumatism or chondrocalcinosis, different amounts of HA and CPPD crystals were demonstrated by Bély and Apáthy’s non-staining technique. In unstained tissue sections of 21 patients more or less HA or CPPD crystal existed side by side in deposits (Tables 1 and 2) (Figures 1 and 2); the HA crystal deposits were accompanied in each patient by more or less deposits of CPPD crystals, whereas CPPD crystal deposits did not occur without HA crystals (surgical specimens might have existed without HA or CPPD crystal deposits). In HE stained sections of 57 tissues samples HA crystals were detected only in one (1.75%; 437/1986), and CPPD were detected in 11 (19.29%) of 57 of surgical specimens (Figures 1 and 2) (Table 2). In unstained sections of 57 tissues samples HA crystals were detected in 33 (57.89%) of 57, and CPPD crystals in 30 (52.63%) of 57 tissue specimens; the synovial membranes were always involved (Figures 1 and 2) (Table 2).
| Staining technics Clinical diagnosis |
HE stained tissue section Prevalence n (%) |
Unstained tissue section Prevalence n (%) |
||
|---|---|---|---|---|
| HA | CPPD | HA | CPPD | |
| Apatite rheumatism | ||||
| Patients n=5 of 21 | 0 of 5 (0%) | 2 of 5 (40.0%) | 5 of 5 (100%) | 5 of 5 (100%) |
| Tissue samples n=17 of 57 | 0 of 17 (0%) | 3 of 17 (17.7%) | 10 of 17 (5.88%) | 10 of 17 (5.88%) |
| Chondrocalcinosis | ||||
| Patients n=16 of 21 | 1 of 16 (6.25%) | 5 of 16 (31.25%) | 16 (100% of 16) | 16 of 16 (100%) |
| Tissue samples n=40 of 57 | 1 of 40 (2.5%) | 8 of 40 (20%) | 23 of 40 (75 %) | 20 of 40 (50%) |
| Total n of patients n=21 | 1 of 21 (4.76%) | 7 of 21 (33.33%) | n=21 of 21 (100%) | n=2121 of 21 (100% |
| Total n of tissue sections n=57 | 1 of 57 (1.75%) | 11 of 57 (19.29%) | n=33 of 57 (57.89%) | n=30 of 57 (52.63%) |
| Note: Variable amounts of HA and CPPD crystals were present in tissue sections (n=57) stained with HE and in unstained ones, viewed under polarized light in patients with clinically diagnosed apatite rheumatism or chondrocalcinosis. | ||||
| Pr n0 /y of surgery of 21 patients with clinical diagnosis of apatite rheumatism (AR) n=5 or chondrocalcinosis (Ch-C) n=16 Surgical specimens (n=57) of operated joins (n=26) | HA (Ca5(PO4)3(OH)) crystal deposits in unstained sections of surgical specimens n=57 |
CPPD (Ca2P2O7.2H2O) crystal deposits in unstained sections of surgical specimens n=57 |
(CaPO4) or (CaCO3) stained with HE, Alizarin red S or von Kossa reaction |
Stained by HE – HA | Stained by HE – CPPD |
|---|---|---|---|---|---|
| 28 tissue samples of 9 patients. with dominant HA crystal deposits | |||||
| (AR) 3495/97* (right knee) prepatellar bursa |
Dominant Clusters and aggregates |
Sporadic | Abundant | 0 | 1 |
| (AR) 3496/97* (left knee) prepatellar bursa |
Dominant Clusters and aggregates |
Sporadic | Abundant | 0 | 1 |
| (AR) 4443-1997 (right knee) synovial membrane, capsule |
Dominant Clusters and aggregates |
Scattered clumps in toto less than 10% |
Abundant | 0 | 1 |
| (AR) V/2170-19 (right knee) synovial membrane, capsule, cartilage |
Dominant Clusters |
Sporadic | Negative | 0 | 0 |
| (AR) V/2171-19 (right shoulder) synovial membrane, capsule, cartilage |
Dominant Clusters |
Sporadic | Negative | 0 | 0 |
| (AR) 181-2019 (right shoulder) Synovial aspirate |
Dominant | Sporadic | Minimal | 0 | 0 |
| (AR) 181-2019 (left shoulder Synovial aspirate |
Dominant | Sporadic | Minimal | 0 | 0 |
| (AR) C17-2012 (right knee) Synovial aspirate |
Dominant | Sporadic | Minimal | 0 | 0 |
| (Ch-C) 2560-1978 (shoulder) synovial membrane, capsule, cartilage |
Dominant | Sporadic | Minimal | 0 | 0 |
| (Ch-C) 676-1981 (elbow) synovial membrane, capsule, bursa |
Dominant | Sporadic | Abundant | 0 | 0 |
| (Ch-C) 2207-1981 (ND) synovial membrane, capsule |
Dominant | Sporadic | Minimal | 0 | 0 |
| (Ch-C) 834-1983 (hip) synovial membrane, capsule, bursa, tendon |
Dominant | Sporadic | Abundant | 0 | 0 |
| (Ch-C) 3097-1985 (left knee) synovial membrane, capsule, cartilage |
Dominant | Sporadic | Abundant | 0 | 0 |
| Fifteen specimens of 6 patients with mixed HA-CPPD crystal deposits | |||||
| (AR) 2417-2014 (right hip) synovial membrane, capsule |
HA: 60% Clusters and aggregates |
CPPD: 40% Scattered focal clumps |
Abundant | 0 | 0 |
| (AR) 604-2015 (right knee) synovial membrane, capsule |
HA: 60% Clusters and aggregates |
CPPD: 40% Scattered focal clumps |
Abundant | 0 | 0 |
| (Ch-C) 3439-1987 (knee) synovial membrane, capsule |
Mixed HA-CPPD disease; HA:70 % | Mixed HA-CPPD disease; CPPD:30 % | Abundant | 0 | 0 |
| (Ch-C) 192-1989 (wrist) synovial membrane |
Mixed HA-CPPD disease; HA:40 % | Mixed HA-CPPD disease; CPPD:60 % | Abundant | 0 | 0 |
| (Ch-C) 169-2006 (left wrist) synovial membrane, capsule |
Mixed HA-CPPD disease; HA:80 % | Mixed HA-CPPD disease; CPPD:20 % | Abundant | 0 | 0 |
| (Ch-C) 581-2007 (right knee) synovial membrane |
Mixed HA-CPPD disease; HA:60 % | Mixed HA-CPPD disease; CPPD:40 % | Abundant | 0 | 1 |
| (Ch-C) 477-2008 (right knee) synovial membrane, capsule, bursa |
Mixed HA-CPPD disease; HA:60 % | Mixed HA-CPPD disease; CPPD:40 % | Minimal | 0 | 0 |
| Fourteen specimens of 6 patients with dominant CPPD crystal deposits | |||||
| (Ch-C) 14-1975 (right hand, wrist) tendon sheets |
Sporadic | Dominant, focal clumps of fragmented crystals | Abundant | 0 | 0 |
| (Ch-C) 631-1980 (left hip) synovial membrane, capsule, cartilage |
Sporadic | Dominant | Abundant | 0 | 0 |
| (Ch-C) 2739-1983 (hip) synovial membrane, capsule, cartilage |
Sporadic | Dominant | Abundant | 0 | 2 |
| (Ch-C) 437-1986 (left hip) synovial membrane, capsule, cartilage |
Sporadic | Dominant | Abundant | 1 | 2 |
| (Ch-C) 3558-2002 (left knee) synovial membrane, capsule |
Sporadic | Dominant (in some foci only fragments) | Abundant | 0 | 2 |
| (Ch-C) 2086-1994 (left knee) synovial membrane |
HA varies 10- 90 % |
Dominant CPPD varies 10-90% |
Abundant | 0 | 1 |
| Note: Apatite Rheumatism (AR) and Chondrocalcinosis (Ch-C) were diagnosed clinically. Twenty-six joints of 21 patients were operated; 57 surgical specimens were analyzed. | |||||
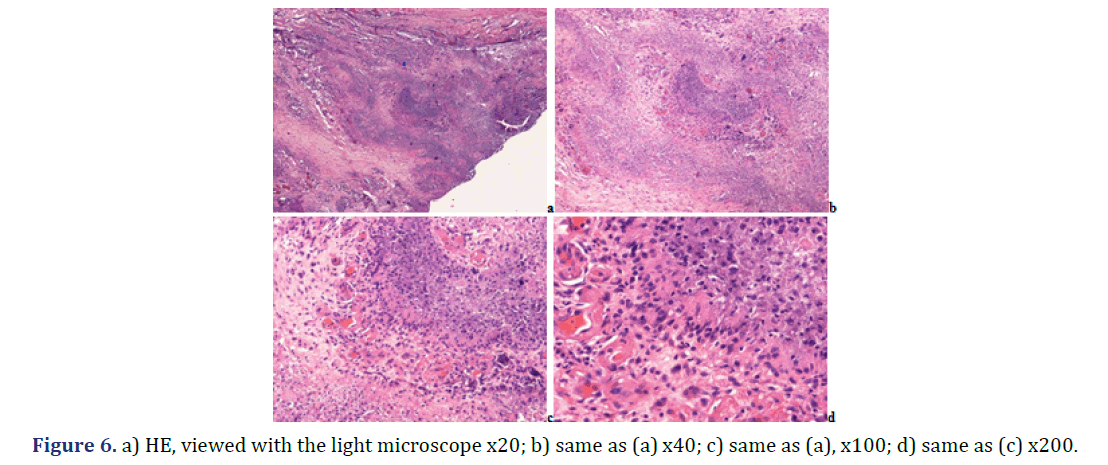
Note: a) HE, viewed with the light microscope x40; b) same as (a) x100; c) same as (a) x200; d) same as b) x200; e) same as
(a), HE, viewed under polarized light x40; f) same as (e) x100; g) same as (e)x200; h) same as (f) x200; i) same tissue section,
unstained, viewed under polarized light, illuminated by 100 watts, x600 High brightness microscopic field is dominated by
HA crystals; j) same field as (i), unstained section, viewed under polarized light, illuminated by 60 watts, x600.
Note: a) HE; viewed with the light microscope x20; b) same as (a) x40; c) same as (a) x100; d) same as (a) x200; e) same as
(a); HE; viewed under polarized light x20; f) same as (b) x40; g) same as (c) x100; h) same as (d) x200; i) same field as (c);
unstained section; viewed under polarized light; illuminated by 100 watts; x100; j) same field as (d) x200; Foci with different
amounts of HA (red arrow head) and CPPD crystals (yellow star) exist side by side; the birefringence of CPPD crystals is
stronger than that of HA crystals; k) same field as (c); unstained sections; viewed under polarized light; using Red I compensator
x100; l) same field as (d) x200.
The HA and CPPD crystal deposits were more frequent and more massive in the synovial membranes (Figures 1 and 2), compared to the synovial capsules, bursae, tendon sheets or cartilages (with or without bones). In 57 tissue samples of 21 patients with the clinical diagnosis of apatite rheumatism or chondrocalcinosis the prevalence of HA or CPPD crystals was more frequent in unstained sections than in the HE stained ones; the difference was significant (c=1, χ2=7.8882, p<0.0049). In contrast to the classic HE stains, the Bély and Apáthy’s non-staining technique was more effective; HA crystals were detected in 33 (57.89%) of 57 tissue samples, and CPPD crystals were detected in 30 (52.63%) of 57 tissue samples of 21 patients with clinically diagnosed apatite rheumatism or chondrocalcinosis.
Table 1 summarizes the occurrence of HA and CPPD crystals in 57 tissue samples of 21 patients with clinical diagnosis of apatite rheumatism or chondrocalcinosis.
HA crystals were detected only in 1 (1.75%) of 57 tissues section (437/1986) stained with HE and viewed under polarized light in patients with clinically diagnosed chondrocalcinosis. HA crystals were detected in unstained sections with Bély and Apathy’s non-staining technique with variable dominance in both patients group; and were present in 33(57.89%) of 57 tissue sections. CPPD crystals were present in 11(19.29%) of 57 tissue sections stained with HE, and in 30(52.63%) of 57 unstained tissue sections.
During fixation, embedding in paraffin or staining with HE the more soluble small and weakly birefringent HA crystals are dissolved, and are virtually non-ex istent (there is no detectable birefringence); the few CPPD crystals have also dissolved in this tissue section and are not detectable. With less brightness, less HA crystals may be detected; the birefringence of CPPD crystals is more dominant.
The solubility of HA and CPPD crystals is different in conventional fix atives (aqueous formaldehyde solution), in ethyl alcohol, acetone, xylene and in aqueous solutions of dyes. The more soluble small and weak birefringent HA crystals are virtually non-existent (there is no detectable birefringence) in the HE stained section, while the less soluble large and strong birefringent CPPD crystals are preserved and demonstrable by polarized light. Under polarized light the direction of birefringence is positive according to the long axis of HA or CPPD crystals. Using Red I compensator the intensity of illumination markedly reduced.
Foci with different amounts of HA and CPPD crystals exist side by side; the birefringence of CPPD crystals is stronger than that of HA crystals.
The strongly birefringent CPPD crystals dominate deceptively the microscopic field, especially using the Red I compensator. The Red I compensator particularly reduce the brightness of illumination deceptively amplifying the presence of CPPD crystals. The HA crystals are colourless, translucent fragments or small (50-500 nm), rod-shaped prisms, with weak birefringence. The CPPD crystals are sub microscopic to 40 μm, rhomboid, strongly birefringent crystals.
The strongly birefringent CPPD crystals dominate deceptively the microscopic field, especially using the Red I compensator. The Red I compensator particularly reduce the brightness of illumination deceptively amplifying the presence of CPPD crystals. Under polarized light the direction of birefringence is positive according to the long axis of HA or CPPD crystals. The weak illumination deceptively amplifying the presence of strongly birefringent CPPD crystals; in contrast to HA crystals.
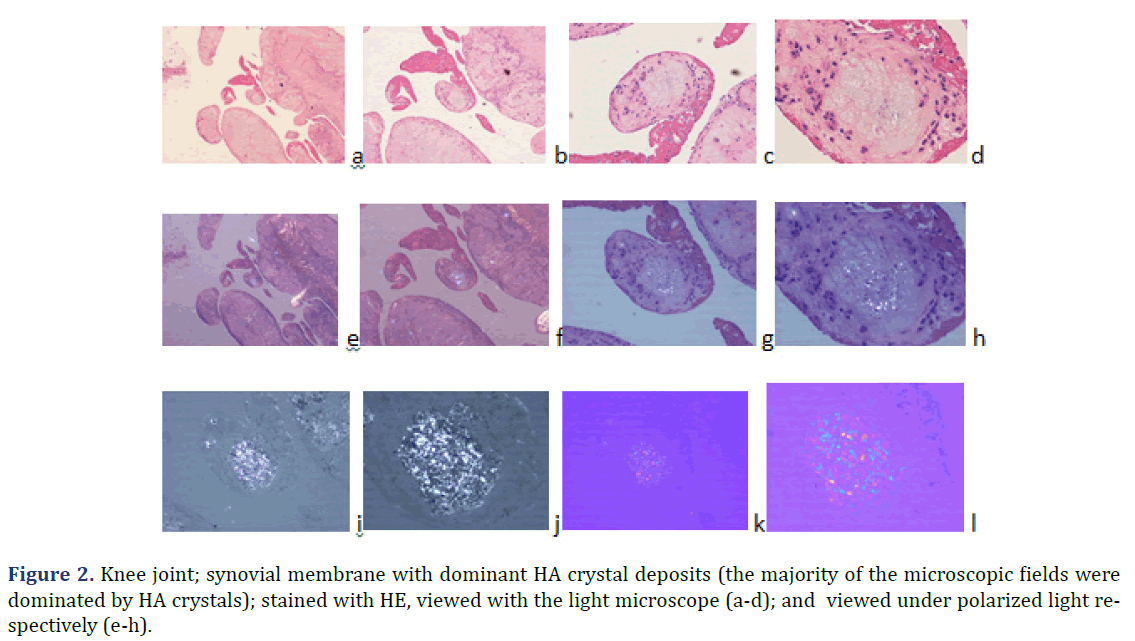
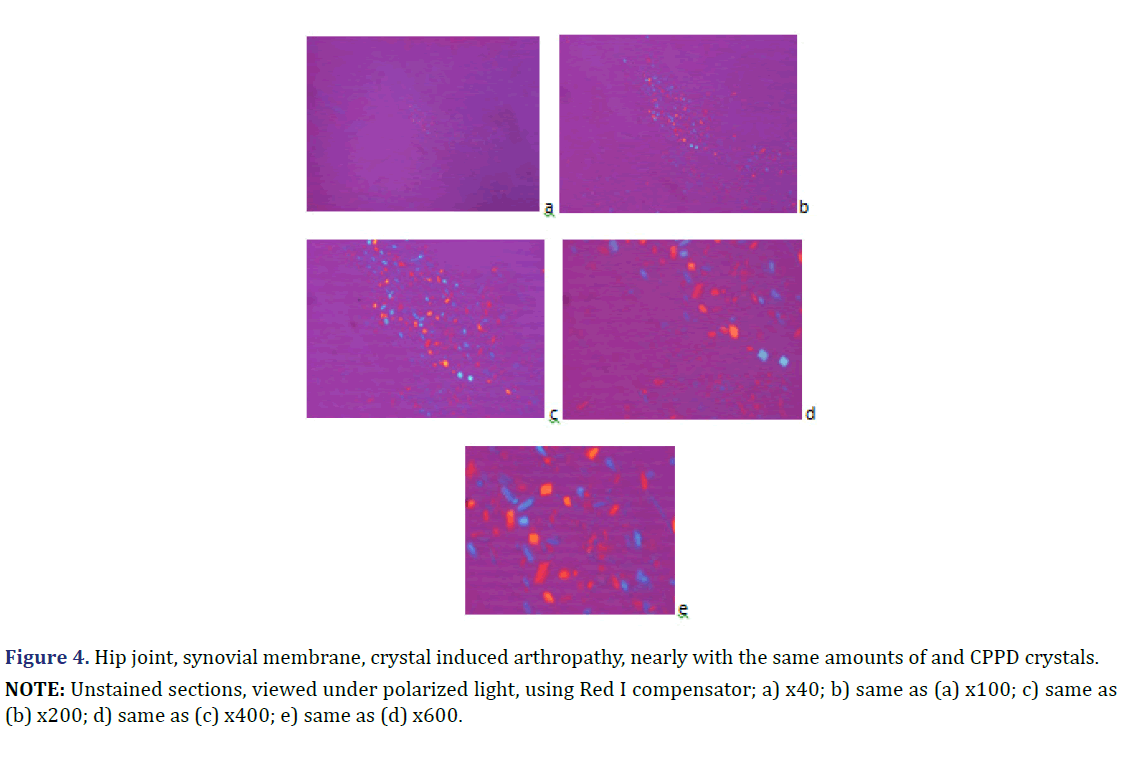
Unstained sections of 57 surgical samples of 21 patients showed more frequent HA crystal depositions than CPPD crystal depositions (the majority of the microscopic fields were dominated by HA crystals rather than by CPPD crystals). HA crystal deposition was dominant (>90% of crystals) in four of 5 patients with the clinical diagnosis of apatite rheumatism, and in five of 16 patients with the clinical diagnosis of chondrocalcinosis (Figures 1 and 2) (Table 2). The amounts of HA and CPPD crystals were nearly the same in one of 5 patients with the clinical diagnosis of apatite rheumatism, and in five of 16 patients with the clinical diagnosis of chondrocalcinosis (Table 3). Finally, more microscopic fields were found with dominant CPPD crystal deposition (>90% of deposited crystals) in six of 16 patients with the clinical diagnosis of chondrocalcinosis (Table 2). The strongly birefringent CPPD crystals appeared deceptively dominant per microscopic fields (Figures 3 and 4). HA crystals were dominant in 28 of 57 tissue samples of nine patients versus CPPD crystals.
| Patients n=21 | Number of surgeries | Mean age in years at surgery ± SD | Range (In years) |
|---|---|---|---|
| Pts. with dominant HA crystal deposits | 9 of 21 | 65.63±15.78 | 39 – 82 |
| Female | 9 | 65.63±15.78 | 39 – 82 |
| Male | 0 | – | – |
| Pts. with mixed HA and CPPD crystal deposits | 6 of 21 | 73.33±8.91 | 59 – 81 |
| Female | 4 | 74.75±10.53 | 59 – 81 |
| Male | 2 | 70.50±6.36 | 66 – 75 |
| Pts. with dominant CPPD crystal deposits | 6 of 21 | 60.67±13.75 | 42 – 75 |
| Female | 5 | 58.20±13.81 | 42 – 75 |
| Male | 1 | 73.00±0.00 | – |
| Note: In unstained sections according to Bély and Apáthy’s technique, the presence of HA crystals was absolutely dominant in 9 (42.86 % of 21) patients (accompanied with a few CPPD crystals). The presence of CPPD and HA crystals was about equal in 6 (28.57 % of 21) patients. The presence of CPPD crystal s was dominant in 6 (28.57 % of 21) patients (in combination with HA crystals). |
|||
NOTE: Unstained sections, viewed under polarized light: a) x40; b) same as (a) x100; c) same as (b) x200; d) same as (c)
x400; e) same as (d) x600.
NOTE: Unstained sections, viewed under polarized light, using Red I compensator; a) x40; b) same as (a) x100; c) same as
(b) x200; d) same as (c) x400; e) same as (d) x600.
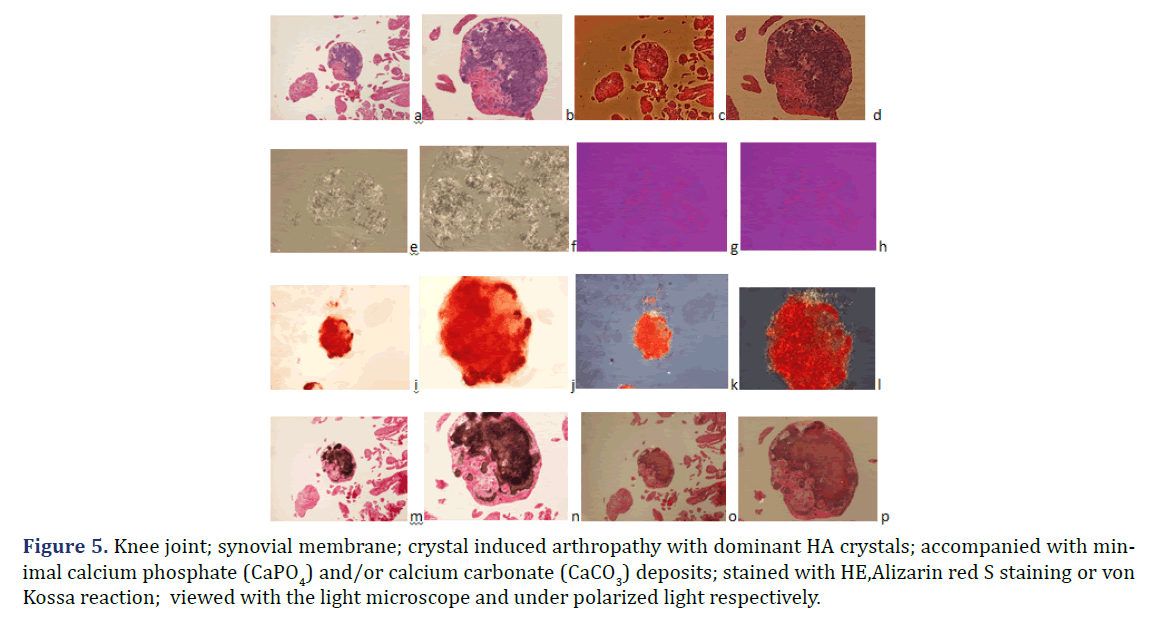
HA crystals were not accompanied by calcium phosphate (CaPO4) and/or calcium carbonate (CaCO3) deposition in six of 28 tissue samples (Figures 1 and 2) the amorphous mineral deposition was minimal in eight (Figure 5), and abundant in further fourteen tissue samples (Table 2). Large amounts (>10%) of CPPD crystals were always accompanied by abundant amorphous calcium phosphate (CaPO4) and/or calcium carbonate (CaCO3) mineral deposits in 24 of 57 tissue samples of twelve patients; in 11 specimens of six patients with mixed HE-CPPD deposits, and in 13 specimens of six patients with dominant CPPD deposits (Table 4).
Figure 5. Knee joint; synovial membrane; crystal induced arthropathy with dominant HA crystals; accompanied with minimal calcium phosphate (CaPO4) and/or calcium carbonate (CaCO3) deposits; stained with HE,Alizarin red S staining or von Kossa reaction; viewed with the light microscope and under polarized light respectively.
| Patient cohorts with dominant HA and/or DPPD deposition | p<0.05 |
|---|---|
| Pts. with dominant HA n=9 of 21 versus dominant CPPD n=6 of 21 | 0.543 |
| Female n=9 of 9 versus n=5 of 6 | 0.394 |
| Male n=0 of 9 versus n=1 of 6 | – |
| Pts. with dominant HA n=9 of 21 versus mixed HA and CPPD n=6 of 21 | 0.271 |
| Female n=9 of 9 versus n=4 of 6 | 0.266 |
| Male n=0 of 9 versus n=2 of 6 | – |
| Pts. with mixed HA and CPPD n=6 of 21 versus dominant CPPD n=6 of 21 | 0.092 |
| Female n=4 of 6 versus n=5 of 6 | 0.081 |
| Male n=2 of 6 versus n=1 of 6 | – |
| Note: There was no significant difference in mean age between patient cohorts with dominant HA, mixed HA and CPPD, furthermore with dominant CPPD deposits. Differences were not calculated between male patients because of the few males. | |
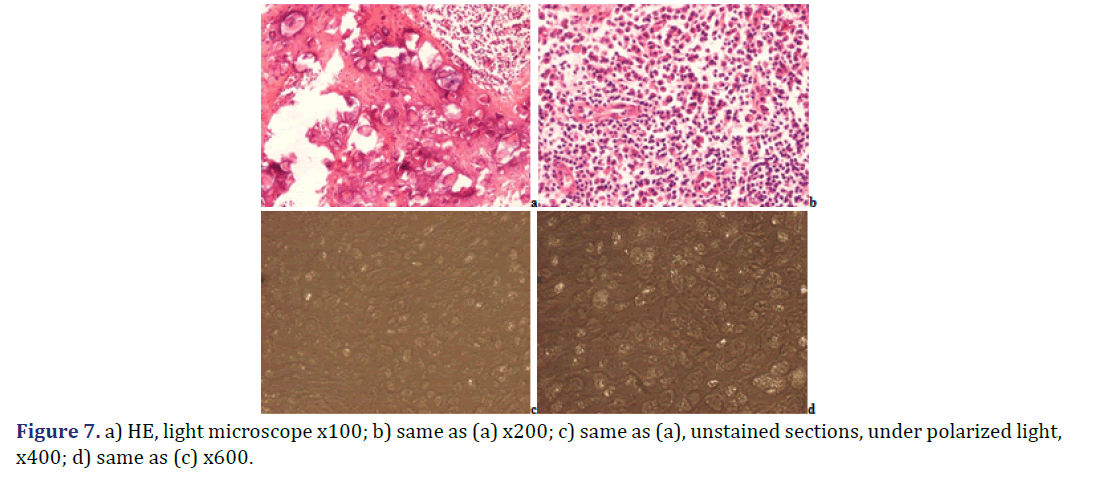
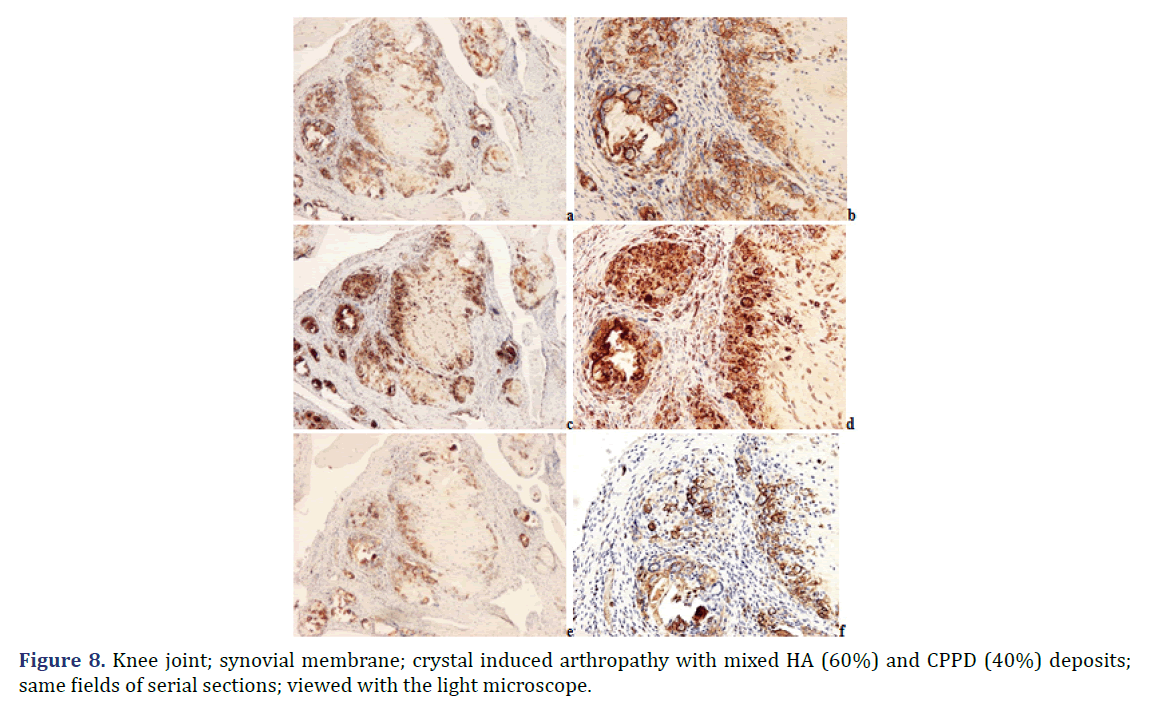
Foci of amorphous mineral deposits in fibrous synovial membrane are associated with acute-subacute inflammation. The HA crystals (with or without CPPD crystals) are accompanied by an intensive acute-subacute inflammatory reaction, including neutrophilic and eosinophilic granulocytes, and monocytes. Phagocytes with intracytoplasmic HA crystals, accompanied by a few phagocytes with strong birefringent CPPD crystal fragments.
Immunohistochemical reaction demonstrates monocytes, macrophages, multinucleate giant cells and T-cells around of HA and CPPD deposits. CD20 and CD79α B-cells were present only sporadically.
2. Patient cohorts with dominant HA and CPPD crystal deposits
Table 2 Summarizes the dominant HA and/or CPPD crystal deposits, furthermore amorphous calcium phosphate or carbonate deposits in surgical specimens of patients with clinically diagnosed Apatite Rheumatism (AR) and CHondro-Calcinosis (Ch-C), comparing the unstained sections with HE stained ones.
HA and CPPD varied within one tissue section. HA (Ca5 (PO4)3(OH)) or CPPD (Ca2P2O7.2H2O) crystal deposits were demonstrated with the non-staining techniques of Bély and Apáthy in all 21 patients. In two cases the HA (Ca5 (PO4)3(OH)) crystals were identified by electron diffraction as well. In unstained tissue sections of 57 surgical specimens HA (Ca5 (PO4)3(OH)) crystals were found in 33, and CPPD (Ca2P2O7.2H2O) crystals in 30 of 57 specimens. In tissue sections stained with HE the HA crystals were found only in one patient (in the synovial membrane of the left hip joint, 437/1986) and CPPD crystals in 7 of 21 patients (in 11 of 57 surgical specimens). The size of individual HA crystals is submicroscopic [39], according to others 50-500 nm [40], in clusters 1-5 μm [40] or 1.9-15.6 μm [41]. In apatite rheumatism the HA crystal clusters “tend to aggregate into globular clumps, and can appear as refractile shiny coins on light microscopy, but show no birefringence under compensated polarized microscopy (400x)” with traditional stains [11]. Using a professional polarizing microscope with high, at least 100-Watt brightness the HA crystal clusters (1-5 μm) are visible and birefringent with polarized light (100x), moreover they can form larger aggregates (conglomerates) of 100 μm or larger, which are visible with an objective of 40x by non-staining techniques [24,25]. The birefringence of HA crystals is weak compared to the strong birefringence of CPPD crystals; the strongly birefringent CPPD crystals may deceptively dominate the microscopic fields (Figures 2-4). CPPD crystals are less soluble in 8% aqueous formaldehyde solution and in aqueous solutions of dyes than HA crystals; variable amounts of CPPD crystals can be occasionally preserved in tissue sections, and remain demonstrable in conventionally fixed tissues, stained with HE, Alizarin red S or von Kossa’s reaction. In unstained sections viewed under polarized light the CPPD crystals have a rhomboid shape (Figures 2-4). The expected size range is 0.42-17.9 μm [38], according to others it varies from sub microscopic to 40 μm [41]. With Red I compensator the CPPD crystals show a strong (Figure 3) positive (Figure 4) birefringence, in contrast to the weak (Figures 2-4) positive (Figure 4) birefringence of HA crystals. Calcium phosphate (CaPO4) and/or Calcium carbonate (CaCO3) deposits were stained with HE, Alizarin red S or von Kossa reaction. Calcium phosphate (CaPO4) and/or Calcium carbonate (CaCO3) deposits may mask the exceptionally preserved HA or occasionally retained CPPD crystals in tissue sections. The crystalline structure of calcium or phosphate does not stain with HE, Alizarin red S or von Kossa reaction, the HA or CPPD crystals remain intact with conventional staining and reaction.
3. Demographics of patients with dominant HA and CPPD crystal deposits
The mean age of patients with dominant HA crystal deposits was higher at the time of surgery, than the mean age of the patients with dominant CPPD deposits (65.63 year versus 60.67 year), but the difference was not significant at an alpha level of 0.05 (p<0.543). The mean age of patients with mixed HA and CPPD crystal deposits was the highest (73.33 year) between our patient groups, without significant differences versus patient groups with dominant HA (p<0.271) or dominant CPPD (p<0.092) crystal deposits. Table 3 Summarizes the demographics of patient cohorts with dominant HA, mixed HA and CPPD, furthermore with dominant CPPD crystal deposits.
Table 4 Summarizes the p” values of significance (at an alpha level of 0.05) comparing the mean age of patients with dominant HA, mixed HA and CPPD, furthermore with dominant CPPD crystal deposition.
Discussion
Apatite rheumatism and chondrocalcinosis are regarded as different metabolic diseases, and distinct clinical entities [1–11]. Great clinical studies, and clinicopathologic hand books support this view, since the HA crystals are virtually not existing in conventional stained tissue sections, while CPPD crystal may be retained (Figures 1 and 2) [5–8].
Introduction of the non-staining technique by Bély and Apáthy opened a new technique in the diagnosis of crystal induced diseases. In non-stained tissue sections of patients with clinically diagnosed apatite rheumatism and chondrocalcinosis, various foci existed side by side in all patients, with different amount of HA and CPPD crystals (Figures 3-5).
The dominance of HA and / or CPPD crystals in tissue sections may justify the use of the old terminology for apatite rheumatism and chondrocalcinosis. Crystal induced metabolic diseases are progressive maladies.
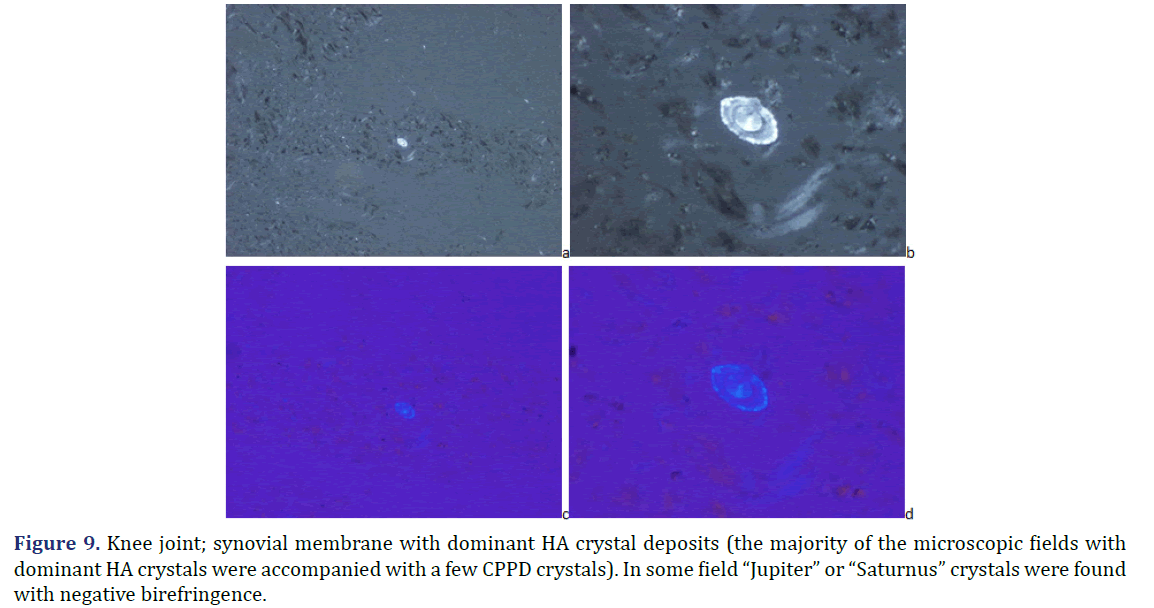
Progressive cumulative pathological processes (like systemic amyloid deposition) begin in organs and on tissue structures that are frequently involved by marked deposits; where deposits are infrequent or less marked, deposition starts later [42–45]. This statement was mathematically confirmed by Ratkó [46], based on the Wald equity [47]. The “most common deposit is the earliest deposit” (the most common is the first) is a fundamental truth for all progressive diseases with cumulative deposition of crystals, proteins, lipids, etc. A disease with progressive cumulative deposition obviously cannot start where no deposition occurs. According to this general rule the HA and/or CPPD crystal deposition starts on the most frequently involved articular or periarticular tissues of the most common involved joint. In our previous study the knee has been the most commonly involved joint in both patient groups with the clinical diagnosis of apatite rheumatism or chondrocalcinosis [29]. The patients in present study had interventions most often due to knee complaints (n=14, 53.85% of 26 surgery), then in descending order the hip (n=5, 19.23%), shoulder (n=4, 15.38%), wrist (n=7.69%), and the elbow (n=1, 3.85%). Unstained sections of 57 surgical samples of 21 patients showed more frequent, and more marked HA crystal deposition (n=33) than CPPD crystal deposition (n=30); more microscopic fields were found with dominant HA crystal deposition than CPPD crystal deposition (Figures 1 and 2). In our interpretation the HA or CPPD crystal induced metabolic diseases start with deposition of HA, followed by the appearance of CPPD crystals. The HA or CPPD crystals provoke an acute inflammatory reaction with polymorphonuclear leucocytes (neutrophilic and eosinophilic granulocytes), monocytes and macrophages (Figures 6-8). HA and CPPD crystals are accompanied by amorphous (CaPO4) or (CaCO3) mineral deposition. The HA crystal deposits were not accompanied by amorphous mineral deposits or in several cases only with minimal ones, while the CPPD crystals were always accompanied by massive (CaPO4) or (CaCO3) deposition; thus presumably the amorphous deposition follows the deposition of HA and CPPD crystals. In our interpretation the deposition of amorphous (CaPO4) or (CaCO3) is a tissue defense mechanism, like fibrosis of soft tissues, leading to a chronic stage of inflammatory reaction of the involved joint. Since the basic metabolic disorder still exists, the pathological processes progress, and involve further joints, leading to a polyarticular progressive malady. In each case, the indication for surgery is determined by the current joint complaints (and this is not necessarily the first affected joint). The progressive nature of metabolic arthropathies is also supported by their clinical stages as well; for chondrocalcinosis: asymptomatic CPP disease (“asymptomatic CPPD”), acute CPP crystal arthritis (“pseudogout”), and chronic CPP crystal inflammatory arthritis (“pseudo-RA”) were recommended [37], and for apatite rheumatism: pre-calcific, formative, resting phases, including resorptive phase characterized by inflammation, and post-calcific phase characterized by reparative processes [38]. With the non-staining technique of Bély and Apáthy several other crystals of different arrangement, shape, size, and quality (intensity and direction) of birefringence may be detected; for exact identification of these crystals electron microscopy, electron diffraction analysis or chemical analytical methods are needed (Figure 9).
Note: (a) anti-human LCA (CD45) diluted (monoclonal antibody N1514; DAKO; Glostrup; Denmark); x40; (b) anti-human LCA (CD45) diluted (monoclonal antibody N1514; DAKO; Glostrup; Denmark) x100; (c) anti-human CD68 (monoclonal antibody N1577; DAKO; Glostrup; Denmark), Streptavidin-biotin-complex/horseradish peroxidase reaction; x40; (d) anti-human CD68 (monoclonal antibody N1577; DAKO; Glostrup; Denmark), Streptavidin-biotin-complex/horseradish peroxidase reaction x100; (e) anti-human CD3(monoclonal antibody RM-9107-R7; Lab Vision; Suffolk; UK); x40; (f) anti-human CD3 (monoclonal antibody RM-9107-R7; Lab Vision; Suffolk; UK) x100
Note: (a) Unstained section; viewed under polarized light; x200; (b) Unstained section; viewed under polarized light; x600; (c) Unstained section; using Red I compensator; viewed under polarized light; x200; (d) Unstained section; using Red I compensator; viewed under polarized light; x600
Frozen sections viewed under polarized light contain large amounts of cholesterol crystals [47]. The large deposits of cholesterol crystals can obscure other crystals and the targeted crystals may be hidden. In suspected crystal induced diseases, the use of fat solvents on frozen sections can be useful to demonstrate other crystals than cholestrol which are less soluble in fat solvents.
Conclusion
Apatite rheumatism and chondrocalcinosis are considered different metabolic diseases and distinct clinical entities. By Bély and Apáthy’s non staining technique different amounts of HA and CPPD crystals were present in both clinically diagnosed metabolic disorders. The dominance of HA and/or CPPD crystal deposits in cartilage, synovial membranes, joint capsules, tendons and periarticular soft tissues may account for the old terminology of apatite rheumatism and chondrocalcinosis. The crystal induced metabolic diseases are progressive cumulative processes which begin on intra- or periarticular tissues that are the most frequently involved (“the most common is the first”).
The more frequent and the more massive HA or CPPD crystal deposits were found in synovial membranes of the knee joint, followed by articular capsules, bursae, tendon sheets and cartilage (with and without bones).
According to our data “apatite rheumatism” and “chondrocalcinosis” are different stages of the same crystal induced metabolic disorder, which are starting with crystal deposition in the synovial membranes of the knee, followed by other joints, with possibly dominant (prevailing) clinical complaints and symptoms at the time of medical attention.
References
- Žitňan D, Sitaj Š. Chondrocalcinosis articularis section L Clinical and radiological study. Ann Rheum Dis 1963; 22:142-152.
- Chondrocalcinosis articularis section L Clinical and radiological study
- Dieppe PA. “Milwaukee shoulder”. Br Med J (Clin Res Ed) 1981; 283: 1488–1489.
[Crossref] [Google scholar] [PubMed].
- Dieppe PA, Doherty M, Macfarlane DG, Hutton CW, Bradfield JW, Watt I. “Apatite associated destructive arthritis”. Rheumatology 1984; 23: 84–91.
[Crossref] [Google scholar] [PubMed].
- Gardner DL, McClure J. “Metabolic nutritional and endocrine diseases of connective tissue, Crystal deposition disease, Calcium pyrophosphate dehydrate crystal deposition disease (chondrocalcinosis; pseudogout), Calcifying tenosynovits, Calcium hydroxyapatite and articular disease”. 1ST edition. 1992.
- Gicht MW. Kalziumpirophosphat-Arthropathie. Apatitkrankheiten “In Gelenkpathologie, historische Grundlagen, Ursachen und Entwicklungen von Gelenkleiden und ihre Pathomorphologie, Berlin, Heidelberg, Springer-Verlag, Germany, Editor: Mohr W. 2000:193-201.
- Reginato AJ, Reginato AM. “Diseases associated with deposition of calcium pyrophosphate or hydroxyapatite” In: Crystal-associated synovitis, Section XV, Kelly’s Textbook of Rheumatology, 6th ed. WB Saunders Company: A division of Harcourt Brace & Company, Philadelphia, London, New York, St. Louis, Sydney, Toronto, Editors: Ruddy Sh, Harris ED jr, Sledge CB, 2001:1377-1390.
- Fassbender H.G. “Crystal induced arthropathies, Calcium pyrophosphate dehydrate crystal deposition disease, Hydroxyapatite crystal deposition disease” In: Pathology and pathobiology of rheumatic diseases (Editor: Fassbender HG), 2nd ed. Berlin, Heidelberg, New York, Springer-Verlag, Germany, 2002:370-379.
- Gupta SJ. “Crystal induced arthritis: An overview”. J Indian Rheumatol Assoc 2002; 10:5-13.
- Rosenthal AK and Ryan LM. “Calcium pyrophosphate deposition disease”. N Engl J Med 2016; 374: 2575-84.
[Crossref] [Google scholar] [PubMed].
- Reginato AM, Yuvienco C. “Hydroxyapatite crystal-induced”. Rheumatology 2017.
- Pseudogout. Mayo Clinic Press 2022.
- Bachmann D, Resnick D. “Cakcium pyrophosphate dihydrate crystal depositin disease” and “Cakcium hydroxyapatite crystal depositin disease”. In: Radilogical atlas of rheumatological diseases (Editors: Bachmann D, Resnick D), Editiones Roche, F. Hoffmann-La Roche Ltd., Basel, Switzerland 1994; 108- 123.
- Garcia GM, McCord GC, Kumar R. “Hydroxyapatite crystal deposition disease”. Semin Musculoskelet Radiol 2003;7:187-93.
- Rosenthal AK, Dalbeth N, Curtis MR. “Clinical manifestations and diagnosis of calcium pyrophosphate crystal deposition (CPPD) disease”. UpToDate 2022.
- Pálinkás M, Poór Gy: “Kristályatrthritisek”. In Reumatológia (Editors: Szekanecz Z, Nagy Gy). Medicina könyvkiadó Zrt. Budapest, 2019:572.
- Bély M, Apáthy Á. “Mönckeberg’s sclerosis – crystal induced angiopathy”. Orvosi Hetilap 2013; 154: 908-13.
[Crossref] [Google scholar] [PubMed].
- Bély M, Krutsay M. ‘’A simple method to demonstrate urate crystals in formalin fixed tissue.” J. autoimmun dis. rheumatol 2013; 1: 46-49.
[Crossref] [Google scholar].
- Bély M, Apáthy A. “Functional role of hydroxyapatite crystals in monckeberg's arteriosclerosis”. J. Cardiovasc. Dis 2014; 2: 228-234.
- Bély M, Apáthy Á. “A microscopic method for identification of calcium pyrophosphate dihydrate deposits in tissues”. Ann. Rheum. Dis 2014.
[Crossref].
- Bély M, Apáthy Ά. “A simple method for the microscopic identification of calcium pyrophosphate dihydrate and hydroxyapatite deposits in metabolic and crystal induced diseases”. Ann. Rheum. Dis 2014; 73:1081.
[Crossref].
- Bély M, Apáthy Ά. ‘’Calcium pyrophosphate dihydrate and hydroxyapatite crystal induced metabolic diseases – same or different?’’ Ann. Rheum. Dis 2016; 75: 1184.
[Crossref] [Google scholar].
- Bély M, Apáthy Á. “A simple method of diagnostic pathology for identification of crystal deposits in metabolic and crystal induced diseases”. Structural Chemistry & Crystallography Communication 2016; 2:1-15.
- Bély M, Apáthy A. “Metabolic diseases and crystal induced arthropathies technic of non-staining histologic sections - A comparative study of standard stains and histochemical reactions”. Clin Arch Bone Joint Dis 2018.
[Crossref] [Google scholar].
- Bély M, Apáthy A. “Crystal deposits in tissue of patients with chondrocalcinosis and apatite rheumatism – Microscopic identification of CPPD and HA with the non-staining technique of Bely and Apáthy”. BAOJ Clinical Trials 2018;4: 018.
- Bély M, Balogh K. “Calcification and crystal deposition of aortic valves in clinically latent metabolic disease – A case report”. EC Cardiology, 2021; 8: 40-47.
- Bély M, Apáthy Á. “Vascular calcification and crystal deposition diseases - A comparative study on 67 amputated legs with atherosclerosis and/or Mönckeberg sclerosis”. EC Cardiology 2021; 8: 01-19.
- Bély M, Apáthy A. ‘’Crystal deposits in primary synovial chondromatosis”. Ann. Rheum. Dis 2022; 81:1642.
[Crossref] [Google scholar].
- Bély M, Apáthy A. “Crystal deposits in apatite rheumatism and chondrocalcinosis – microscopic identification of hydroxyapatite and calcium pyrophosphate dihydrate crystals with standard stains and histochemical reactions and with the nonstinging technique of Bély and Apáthy”. EC Pulmonology and Respiratory Medicine, 2022; 11.5: 03-24.
- Bély M, Apáthy A. ‘’Crystal deposits in tissue of 64 patients with gout – A comparative study of standard stains and histochemical reactions with the non-staining technique of Bély and Apáthy”. EC Cardiology 2022.
- Carson FL. “Mayer’s hematoxylin” In: Histotechnology (Editor: Carson FL), ASCP Press: Chicago 1990; 100-103.
- McManus JFA, Mowry RW. “Methods of general utility for the routine study of tissues”, “Sodium Alizarin sulfonate stain for calcium” and “Von Kossa’s method for phosphates and carbonates”. In: Staining methods, histologic and histochemical 1960; 55-194.
- Vacca LL. “Alizarin red S” In: Laboratory Manual of Histochemistry 1985;333-334.
- Lillie RD: “Von Kóssa’s method” In: Histpathologic Technic and Practical Histochemistry (Editor: Lillie RD), The Blakiston Division McGraw-Hill Book Company, New York, Toronto, London, 1954;264-265.
- Crossref
- Lentner C. “Statistical methods” In Geigy scientific tables, 8th revised and enlarged ed: Ciba-Geigy Limited, Basle, Switzerland, Editor: Lentner C, Compiled by: Diem K, Seldrup J, 1982;227.
- Rosenthal A, Dalbeth N, Curtis MR. ‘’Clinical manifestations and diagnosis of calcium pyrophosphate crystal deposition (CPPD) disease”. UpToDate.
- Uhthoff HK, Loehr JW. "Calcific tendinopathy of the rotator cuff: Pathogenesis, diagnosis, and management". J Am Acad Orthop Surg 1997;5: 183–19.
[Crossref] [Google scholar] [PubMed].
- Swan A, Chapman B, Heap P, Dieppe P, Seward H. “Submicroscopic crystals in osteoarthritic synovial fluids”. Ann Rheum Dis 1994; 53: 467–70.
[Crossref] [Google scholar] [PubMed].
- Pay S, Terkeltaub R. “Calcium pyrophosphate dihydrate and hydroxyapatite crystal deposition in the joint: New developments relevant to the clinician”. Curr Rheumatol Rep 2003; 5.3: 235-43.
- Gatter RA, Schumacher HR. “Microscopic findings under compensated polarized light and phase light”. In: A practical handbook of joint synovial fluid analysis. 2nd edition. 1991.
- Bely M, Apathy A, Pinter T, Ratko J. “Generalized secondary amyloidosis in rheumatoid arthritis” Acta Morphol Hung 1992;40:49-69.
[Google scholar] [PubMed].
- Bély M. “Identification of amyloid deposits by histochemical methods of romhányi and wright: a comparative histochemical and immunohistochemical study”. Amyloid 2001;8:177-182.
- Bély M, Apáthy Á. Clinical pathology of rheumatoid arthritis: Cause of death, lethal complications and associated diseases in rheumatoid arthritis. First English edition. 2012.
- Bély M, Apáthy Á. “Formal pathogenesis of systemic and localized amyloidosis”. EC Cardiology 2019; 6:444-469.
- Ratko I. “Special mathematical and computer science methods in the medical sciences”. 12th International Congress of Cybernetics, Namur. 1989.
- Wald A: Sequential analysis. Wiley mathematical statistics series, Chapman & Hall, New York 1947.
- Balogh K: (Personal communication, 2013).
Copyright: © 2022 The Authors. This is an open access article under the terms of the Creative Commons Attribution NonCommercial ShareAlike 4.0 (https://creativecommons.org/licenses/by-nc-sa/4.0/). This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.