Full Length Research Article - Journal of Interdisciplinary Histopathology (2022)
Epstein Barr positive Diffuse Large Cell Non‐Hodgkin Lymphoma, NOS: A Distinct Morphological Entity that can Easily be Confused with Other Types of Lymphoma
Mario Murguia‐Perez1*, Yunuen I. Garcia‐Mendoza2, Karen S. Vázquez‐Rodríguez2, Rafael Fosado‐Ramos2, Yara G. Flores Raymundo2, Rita Dorantes‐Heredia3, Saulo Mendoza‐Ramírez4, Eduardo Agustín‐Godínez5, Jazmín A. González‐Mercado6, Karen A. Leal‐Tapia7, Alicia G. Siordia‐Reyes7, Octavio Martínez‐Villegas8, Norma E. Alatoma‐Medina8 and Marco A. Olvera‐Olvera12UMAE Speciality Hospital N° 1 National Medical Center Bajío, University of Guanajuato, Guanajuato, Mexico
3Medica Sur Medical Center, National Autonomous University of Mexico, México, Mexico
4ABC Medical Center, National Autonomous University of Mexico, México, Mexico
5General Hospital 450 Durango Health Services, Juarez Durango State University, Durango, Mexico
6Maternal and Child Hospital Durango Health Services, Juarez Durango State University, Durango, Mexico
7UMAE Pediatric Hospital “Dr. Silvertre Frenk Freud” XXI Century National Medical Center, National Autonomous University of Mexico, Mexico City, Mexico
8UMAE Hospital of Gynecology and Pediatrics N° 48 National Medical Center Bajio, University of Guanajuato, Guanajuato, Mexico
Mario Murguia‐Perez, UMAE Speciality Hospital N° 1 National Medical Center Bajío, University of Guanajuato, Guanajuato, Mexico, Email: drmariopatologia@gmail.com
Received: 08-Jun-2022, Manuscript No. EJMJIH-22-66142; Editor assigned: 10-Jun-2022, Pre QC No. EJMJIH-22-66142 (PQ); Reviewed: 24-Jun-2022, QC No. EJMJIH-22-66142; Revised: 29-Jun-2022, Manuscript No. EJMJIH-22-66142 (R); Published: 08-Jul-2022
Abstract
Epstein Barr Virus positive (EBV+) Diffuse Large B-Cell Lymphoma (DLBCL) Non- Special Type (NOS) is an entity included in the 2016 WHO classification of lymphoid neoplasms. Formerly known as “elderly lymphoma”, it is an aggressive B-cell lymphoma associated with chronic EBV infection and a poor prognosis with standard chemotherapeutic approaches. Currently, it is recognized that it can occur in children and young patients. Because of its very low frequency, it causes common errors in its diagnosis, as it is morphologically confused with other types of lymphomas. We present a series of cases in patients from various hospitals in Mexico, including clinical data, immunohistochemical studies, and all with the presence of EBV. Finally, we carried out a discussion about this entity to understand its pathogenesis.
Keywords
Large B-cell lymphoma; Epstein Barr Virus (EBV); immunohistochemistry
Introduction
Diffuse Large B-Cell Lymphomas (DLBCL) is the most common type of lymphomas, accounting for 30-75% in different reports. In the 4th edition of the World Health Organization (WHO) classification of tumors of hematopoietic and lymphoid tissues included a new entity, “EBV+ DLBCL in the elderly (EBV+)” [1], which was defined as an EBV+ DLBCL of patients older than 50 years, who did not have previous lymphoma or underlying immunosuppression [2,3]. Since the initial description of 22 elderly EBV+ DLBCL patients in 2003 by Oyama et al. [4], knowledge of EBV mediated mechanisms of oncogenesis, microRNA (miRNA) profiles in EBV+ lymphomas, and new effective therapies for patients with this neoplasm have developed. More recently, this entity has been identified in younger and immunocompetent patients, without association with poor evolution. This information has led to the revised entity, EBV+ DLBCL Not Otherwise Specified (NOS) (EBV+ DLBCL-NOS) in 2016 WHO criteria [1,5], and whose morphological resemblance to other entities more frequent in that age, such as Classical Hodgkin’s Lymphoma (CHL) and DLBCL rich in T cells and histiocytes (TH-DLBCL), caused disease progression and delayed treatment [5].
Due to the rarity and variable distribution of EBV+ DLBCL-NOS, its pathogenesis is still unclear, especially in the field of genomics worldwide. This has also prevented the treatment of EBV+ DLBCL-NOS. With the advancement of Next-Generation Sequencing (NGS) technology, the molecular composition and function of various malignancies including EBV+ DLBCL-NOS, has been comprehensively and efficiently analyzed. Some studies have shown that EBV+ DLBCL-NOS is characterized by activation of the JAK-STAT and NF-kB pathways. While some mutations in CD79B and CARD11 can be detected, mutations in MYD88 are not detected. Copy Number Alterations (CNA) and gene expression profiles showed that relatively few genomic alterations were found in EBV+ DLBCL-NOS compared to EBV-DLBCL. However, the genomic profile of EBV+ DLBCL-NOS is still unclear [6].
EBV+DLBCL-NOS has been observed worldwide, with a distinct incidence between 2-15% of all DLBCL; it is more prevalent in Asian populations (8 to 15%), followed by Latin America (7%), and is rare in Western countries (<5%) [1,6]. In Mexico, the number of cases is very low, which may be due to a lack of knowledge of the entity, its low suspicion of young patients, and the lack of immunohistochemical equipment in hospitals. Because of the documented challenges, one of us (MM-P) decided to collect cases of EBV+ DLBCL-NOS from different hospitals in the country, managing to collect 6 cases. They gave rise to serious diagnostic problems, and a variety of malignancies were considered in all of t hem. Therefore, we believe it is worthwhile to provide a detailed clinical, morphological, immunohistochemical, and molecular characterization of this under recognized entity.
Materials and Methods
The 6 cases were collected as follows: 2 cases were obtained at UMAE Specialty Hospital No. 1 National Medical Center Bajío, in León, Guanajuato, Mexico; 3 cases were from the consultation files of one of (MM-P), one from the General Hospital 450 Durango Health Services, the sec - ond from León Regional General Hospital, and the third from the author’s private practice. Finally, the sixth case was collected from Medica Sur Foundation (Mexico City), courtesy of one of our co-authors (RD-H). Clinical information was obtained in three cases from the referral hospitals, and in three cases from the referring pathologists. All cases had hematoxylin and eosin sections at 4 micrometers thickness and a complete immunohistochemical battery that confirmed the diagnosis of EBV+ DLBCL-NOS, which are specified in Table 1. The immunohistochemistry of the cases reviewed and consulted in León, Guanajuato was performed on an automated Pathcom Slide Stainer SSI System (Pathcom Inc., Santa Bárbara, CA); while the immunohistochemistry of the case sent from Mexico City used the standard manual technique of peroxidase with avidin-biotin complex and antigen retrieval of epitopes induced by heat. All cases were evaluated by (MM-P), one was jointly reviewed by two authors (MM-P. and RD-H), and another by three authors (MM-P, AGS-R, KAL-T.). In all cases, the clinical follow-up was updated.
| Antibody | Dilution | Source |
|---|---|---|
| CD20 | 1:100 | Diagnostic Biosystem |
| CD3 | 1:50 | Zeta |
| CD15 | 1:50 | GeneAb |
| CD30 | 1:50 | Diagnostic Biosystem |
| EBV (LMP-1) | 1:100 | Zeta |
| ALK-1 | 1:100 | Diagnostic Biosystem |
| MUM-1 | 1:100 | Zeta |
| CD10 | 1:50 | Diagnostic Biosystem |
| BCL6 | 1:100 | Diagnostic Biosystem |
| BCL2 | 1:50 | Diagnostic Biosystem |
| CD68 | 1:100 | Diagnostic Biosystem |
| FASCINA | 1:100 | Diagnostic Biosystem |
Results
Clinical findings
The clinical findings of the patients are presented in Table 2. Three patients were children (2 females and 1 male, aged 4, 6, and 7 years, respectively), 1 patient was a teenager (female, 14 years old) and the remaining 2 patients were older adults (1 woman and 1 man, 54 and 61 years old). All patients presented B symptoms (not specified in) (Table 2), such as weight loss, fever, and night sweats. All presented lymphadenopathies; most frequently the cervical lymph nodes were affected, followed by the mediastinal, and retroperitoneal, among others. At the time of initial diagnosis, they only presented lymphadenopathy. At the time of consultation of the cases, five out of six cases presented extranodal involvement, with the most frequently affected sites being the bone marrow, liver, lung, bone, and skin. Three cases were initially diagnosed as Classical Hodgkin’s Lymphoma (CHL) (3 nodular sclerosis type and 1 lymphoid depletion), one was diagnosed as DLBCL, NOS, another as DLBCL rich in T lymphocytes and histiocytes (TH-DLBCL), and only one was correctly diagnosed from the first pathological approach. Initially, 3 patients were treated with the ABVD scheme for LCH, except for cases 1 and 5, who from the beginning received rituximab, cytarabine, hydrocortisone, and methotrexate. After the review, all patients received a treatment scheme for DLBCL, except for case 1, who died at the time the case was consulted. Case 2 also received full-body radiotherapy. 2 patients died due to extension of the disease (cases 1 and 3), and 3 are alive with disease, clinically stable, and without progression; these patients also received anti- CD30 therapy (brentuximab). Case 6 is a recent diagnosis, so the therapeutic scheme had not yet been started.
| Case No | Age (yrs) | Gender | Immunosupresion | Affected lymph nodes | Extranodal affection | Original diagnosis | Treatment | Follow-up |
|---|---|---|---|---|---|---|---|---|
| 1 | 54 | F | NO | Cervical bilateral, mediastinal, retroperitoneal | Liver, bone marrow | DLCBL, NOS | Metotrexate Citarabine Hidrocortisone Rituximab |
Deceased |
| 2 | 7 | F | YES | Cervical bilateral, supraclavicular bilateral, left axilar, mediastinal | Lung, mesentery | CHL-LD | Rituximab Ifosfamide Carboplatimun Etopositum Body corporal total radioterapy |
LWD |
| 3 | 6 | M | NO | Mediastinal, abdominal, retroperitoneal, paraaortic, pelvic | Bone marrow | CHL-NS | Metotrexate Citarabine Hidrocortisone Rituximab |
Deceased |
| 4 | 14 | F | NO | Cervical bilateral, axilar, mediastinal, retroperitoneal, inguinal | Liver, lung, bone (ribs, bilateral femur, vertebras), bone marrow | TH-DLCBL | Metotrexate Citarabine Hidrocortisone Rituximab Brentuximab |
LWD |
| 5 | 61 | M | NO | Cervical bilateral, mediastinal, retroperitoneal | Skin, bone marrow | CHL-MC | Metotrexate Citarabine Hidrocortisone Rituximab Brentuximab |
LWD |
| 6 | 4 | M | NO | Right cervical nodes | No | EBV+ DLCBL, NOS | None | LWD |
Morphological and immunophenotypic findings
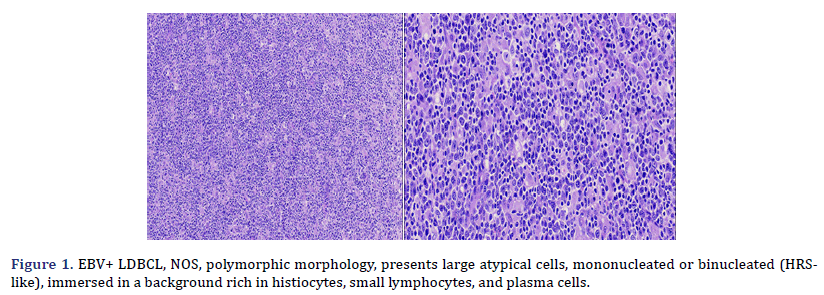
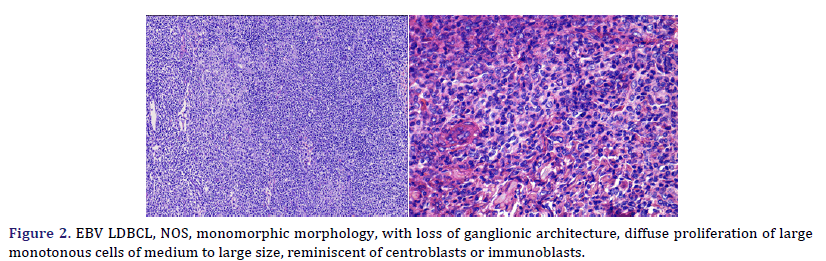
Histological and immunophenotypic characteristics are shown in Table 3. Cases 2, 3, 4, 5, and 6 presented a polymorphic morphology, characterized by the presence of large atypical, mononucleated, or binucleated (HRS-like) cells, immersed in a background rich in histiocytes, small lymphocytes, and plasma cells (Figure 1); two cases also showed eosinophils in varying amounts. Case 1 presented monomorphic morphology, characterized by blurring of the ganglion architecture, secondary to a diffuse proliferation of large monomorphic cells of medium to large size (Figure 2); neoplastic cells resembled centroblasts or immunoblasts; accompanied by partial fibrous septa. Cases 1 and 3 presented extensive geographic necrosis, and 4 of 5 cases showed a high fraction of apoptosis. Case 3 showed prominent perivascular infiltration, angiocentricity, and angioinvasion. Finally, in case 2, the infiltration was accompanied by numerous histiocytes that presented hemophagocytosis in the mediastinal lymph nodes and the lung.
| Case No | Subtype | Necrosis | Apoptosis | CD20 | CD30 | EBV/LMP1 | CD15 | CD10 | BCL2 | BCL6 | MUM1 | KI67 | GCB/ABC |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Mono | Yes | Yes | 3+ | 3+ | 3+ | N | N | N | 3+ | 3+ | 70% | ABC |
| 2 | Poly | No | Yes | 3+ | 3+ | 3+ | 1+ | N | 2+ | 3+ | 3+ | 50% | ABC |
| 3 | Poly | No | Yes | 3+ | 3+ | 3+ | N | 3+ | N | N | N | 90% | GCB |
| 4 | Poly | No | No | 3+ | 3+ | 3+ | 3+ | N | 3+ | 3+ | 3+ | 30% | ABC |
| 5 | Poly | No | No | 3+ | 3+ | 3+ | 2+ | N | N | 3+ | 3+ | 50% | ABC |
| 6 | Poly | No | No | 3+ | 3+ | 3+ | N | N | 3+ | 2+ | 3+ | 70% | ABC |
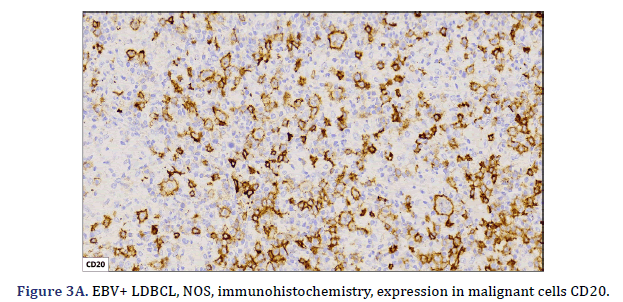
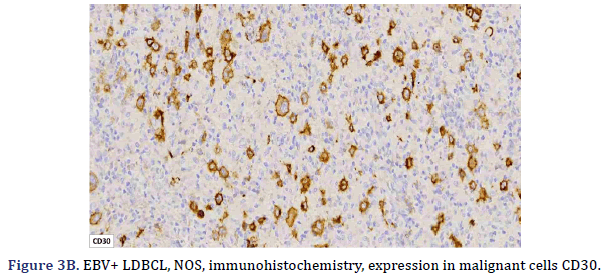
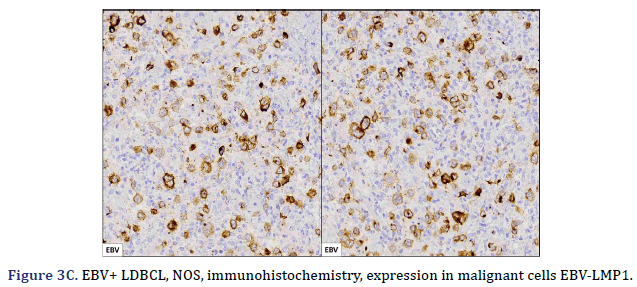
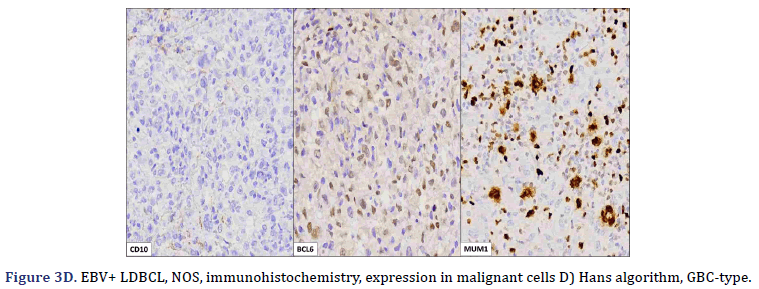
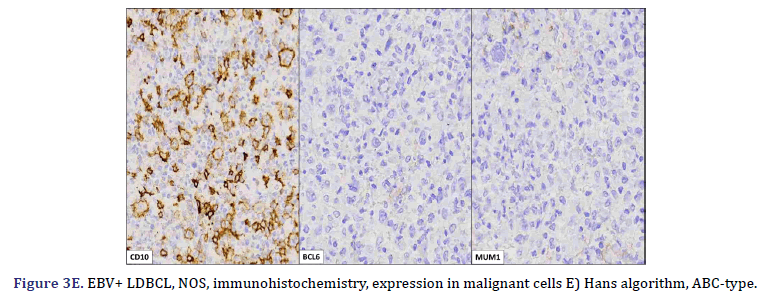
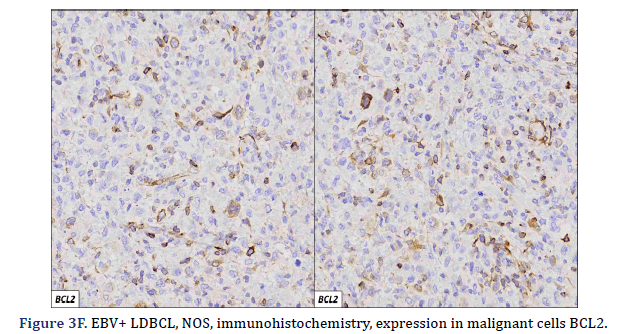
All cases expressed CD20 (Figure 3A), CD30 (Figure 3B), and EBV (Figure 3C) confirmed with expression for EBV/LMP1, with intense expression in neoplastic cells. The cases were classified as germinal center B-Cell (GCB) and activated B cell (ABC) phenotype according to the Hans algorithm; five patients were of the ABC phenotype (Figure 3D), and only case 3 was classified as the GCB phenotype (Figure 3E). BCL2 expression was found in 2 cases (Figure 3F). In case 2, the presence of histiocytes with the expression of CD68 and fascin was demonstrated, the latter expressing in histiocytes and follicular and interdigitating dendritic cells. All cases were negative for ALK-1.
Discussion
EBV+ DLBCL-NOS was first described by Oyama et al. in 2003 [4,7], who reported on 22 senile patients who had dismal clinical outcomes and poor response to therapeutic regimens. Additional retrospective clinicopathological studies and case series led to the introduction of the provisional entity EBV+ DLBCL of the elderly in the 2008 version of the WHO classification of tumors of hematopoietic and lymphoid tissues. Later studies further refined the perception of EBV+ DLBCL due to its occasional appearance in young immunocompetent individuals with evidence of a tolerogenic immune environment [5,8]. All these findings and observations culminated in the adaptation of the WHO definition of the entity in the revised 4th edition of the WHO classification 2016 and renaming it as EBV+ DLBCL-NOS, and without age restriction [1]. The designation “NOS” was added to emphasize the exclusion of the more specific types of EBV associated lymphomas (lymphomatoid granulomatosis, plasmablastic lymphoma, DLBCL associated with chronic inflammation, and EBV associated mucocutaneous ulcer). In our work, we found 4 cases in young patients (3 of them children) and only 2 in adults. This may be since in Mexico the prevalence of EBV infection is high, and it is normally found in the latent phase, which is mentioned below.
Among DLBCL, the prevalence of EBV+ DLBCL-NOS is low in Western countries (4.4% to 5.8%) and slightly higher in Asian and Latin American countries (4.7% to 28%); only 1 studies reported 7% of cases in South America. Africa. In Latin America; Mexico, Peru, and Argentina, all developing populations where EBV infects young children mostly without symptoms, the prevalence of EBV+ DLBCL-NOS in adults reached 7%, 15%, and 9%, respectively [9]. The pathological features have been mainly described as 2 distinct patterns, namely monomorphic, resembling DLBCL, and polymorphic, more closely related to TH-DLBCL, but clinical and outcome correlations with these patterns have not been fully established. The lack of uniform criteria and heterogeneous geographic distribution may explain the reported differences in disease prevalence and prognostic characteristics. The outcome data for EBV+ DLBCL-NOS compared to EBV- DLBCL-NOS remains a matter of debate; some series argue that EBV positivity is associated with poor prognosis, while others suggest that it does not affect survival. Additional work demonstrated that the prognostic impact of EBV positivity could be related to age, with a favorable prognosis in elderly patients, but not in young patients [10].
Clinically, in addition to common nodal involvement and high International Prognostic Index (IPI) scores, patients tend to have high rates of extranodal involvement, with the gastrointestinal tract, skin, and bone marrow being the most commonly affected sites. In addition, there are a higher proportion of patients with elevated LDH levels and a more advanced clinical stage, as well as a worse functional status than patients with EBV+ DLBCL. 5 of the 6 cases studied in this series presented extranodal involvement, with predominance in bone marrow, liver, lung, bone, and skin.
Immunosenescence is a process associated with physiological aging characterized by a series of changes in the function of the immune system. Dysregulation of T cell response, thymic atrophy, reduced production of new T cells, development of anergic memory cells, loss of immunosurveillance, and deficiencies in cytokine production, as well as limitations in the T cell receptor repertoire, are processes that have been associated with immunosenescence. Such processes could be accelerated in the setting of chronic infections such as EBV infection. Given the development of EBV+ DLBCL-NOS in younger individuals without evidence of immunosuppression, other factors may also play a role in the pathogenesis of EBV+ DLBCL- NOS [10-12].
EBV, formally designated gamma Human Herpes Virus type 4 (HHV-4), deserves special attention because it persistently infects most adult humans worldwide. In a literature review and meta-analysis study by Hwang et al., the results showed that the pooled proportion of EBER positivity was 7.9% in patients with de novo DLBCL. The prevalence of EBV+ DLBCL-NOS was significantly higher in Asia and South America compared to Western countries. In addition, a trend to lower pooled proportions was observed in studies that used a higher cut-off point for EBER positivity; the age of the patients did not significantly affect the prevalence [13]. These findings may improve our current understanding of EBV+ DLBCL-NOS. The virus is transmitted through saliva, and transmission begins with the infection of the epithelial cells of the oropharynx, where EBV replicates and reaches local B cells to establish latent infection. Only a small fraction of people infected for life will develop EBV associated cancer. Nevertheless, it is well established that EBV is involved in the etiopathogenesis of a variety of human cancers, in particular the endemic forms of Burkitt’s Lymphomas (BL) and Nasopharyngeal Carcinomas (NPC). The virus is also readily detected within neoplastic cells in a variable number of cases in other malignancies, notably Classical Hodgkin’s Lymphomas (CHL), Non-Hodgkin’s Lymphomas (NHL), and a small fraction of Gastric adeno Carcinomas (GaC) [14-16]. Most neoplastic cells are latently infected in EBV associated neoplasms and express only a subset of viral products. Based on the repertoire of expressed latent viral products, three main EBV latency programs are typically described, known as viral latency types I, II, and III. All latent EBV products are expressed in type III latency, including six EBV Nuclear Antigens (EBNA 1, 2, 3A, 3B, 3C, and LP), three latent membrane proteins (LMP 1, 2A, and 2B), and noxious viruses. Viral Coding RNAs, such as the small RNAs encoded by EBV (EBER 1 and 2) and miR-BARTs. Type II latency is more restricted, featuring an expression of the EBV oncoproteins EBNA1, LMP1, and LMP2A, while most viral products are repressed in type I latency, characterized by expression of EBNA1 and EBER only. These viral latency expression profiles are observed in vivo in latently infected non-neoplastic cells and are also recapitulated in EBV infected malignant cells in cancers [14,16,17].
Two subtypes of EBV+ DLBCL-NOS were identified. The most frequent polymorphic subtype, in which medium neoplastic cells with HRS-like cells are distributed in a reactive background with histiocytes, lymphocytes, and plasma cells. In contrast, the monomorphic subtype is characterized by large neoplastic cells with a centroblastic or immunoblastic morphology, without a polymorphic inflammatory history. Large areas of geographic necrosis or apoptosis can be seen in both subtypes. 5 of the 6 cases evaluated in our series were polymorphic. Beltrán et al., suggested subdividing the polymorphic type into 3 subtypes: polymorphic DLBCL-like, CHL-like, and peripheral T-cell non-Hodgkin’s lymphoma-like (PTPL) [12]. In EBV+ DLBCL-NOS, the neoplastic cells are usually positive for the B-cell antigens CD19, CD20, CD22, CD79a, and PAX5. Furthermore, CD30 is frequently positive and CD15 is co-expressed in a few cases. In EBV+ DLBCL-NOS, activation of the JAK/STAT and NF-kB pathways, which trigger the expression of phosphorylated STAT3 and NF-kB, is more frequent compared to EBV- DLBCL. Approximately 60% of cases show clonal immunoglobulin gene rearrangement and clonality. The evaluation is useful in discriminating polymorphic cases from reactive hyperplasia [9]. Studies using recombinant forms of EBV have shown the absolute requirement for LMP1 in the transformation process. All our cases expressed LMP1. This degree of positivity is in contrast to what is typically seen in CHL and other EBV+ B-cell lymphoproliferative disorders, in which generally only a subset of tumor cells are positive. LMP1 also plays a role in the common expression of the ABC/ non-GCB phenotype and the PD-L1 and CD30 positivity seen in most EBV+ lymphomas. All our cases expressed CD30, but the expression of this activation marker did not predict an adverse outcome in young patients, as suggested in older patients with EBV+ DLBCL-NOS, although in our series, two patients (cases 1 and 3) died, this was due to disease progression from initial misdiagnosis, rather than CD30 expression. LMP1 is also associated with the downregulation of BCL6 [5]. Additionally, genetic mouse models have shown that LMP1 expression is sufficient to model the main features of EBV infection in mice, namely immunogenicity and tumorigenesis, even though EBV is endemic to humans. This is of particular interest because T cell-mediated immune surveillance of LMP1+ B cells contrasts with the belief that the human immune system has evolved to prevent the expansion of EBV infected B cells. The virus could have evolved to be recognized by the mammalian immune system, probably because the lifelong latent infection is advantageous over fatal infection [18]. Using the Hans algorithm, 5 of the 6 cases presented are of the Active B Cell type (ABC), and only one is of the Germinal Center B-cell type (GCB). According to Hans’ algorithm, the ABC-associated IRF4 protein, MUM1, is typically positive, while the Germinal Center B-cell markers (GCB), CD10, and BCL6 are typically negative. Consistent with this, gene expression analyzes have confirmed the prevalence of ABC in EBV associated cases, even in children. However, in various populations, no significant differences in EBV+ DLBCL-NOS were observed between the GCB and ABC subtypes. The predominance of ABC may be caused by the latent viral protein LMP1, expressed in both latency II and III patterns, which mimics a constitutively activated CD40 receptor, which in turn induces the activation of the NF-kB/IRF4 pathway, giving rise to thus to deregulation of BCL6. Furthermore, gene expression profiling in EBV+ DLBCL-NOS revealed that the JAK-STAT and NF-kB pathways were enriched in EBV+ DLBCL-NOS, and this finding was confirmed in vitro in cell lines and also in patients by immunohistochemical staining [9].
Wang et al., found that IL-21 increases the expression of host MYC and AP-1 (composed of related Jun and Fos family proteins) and STAT3 phosphorylation, as well as the expression of the viral protein LMP1. These proteins are known to promote the G1/S phase transition to accelerate cell cycle progression. Furthermore, in NOD/SCID mouse xenograft model experiments, we found that IL-21 treatment increases glucose uptake and angiogenesis in EBV+ DLBCL. Although more samples are needed to validate these observations, our study reconfirms the adverse effects of IL-21 on EBV+ DLBCL-NOS, which has implications for the drug development of DLBCL [19].
The spectrum of B-cell neoplasms associated with EBV infection has increased exponentially, and before a diagnosis of EBV+ DLBCL-NOS is made, there are several entities to consider, therefore, in many cases, EBV+ DLBCL-NOS is a diagnosis of exclusion. The entities to be excluded are those with apparent immunosuppression such as patients with Post-Transplant Lymph proliferative Disorders (PTLD), and patients with iatrogenic induced immunosuppression as those receiving methotrexate, tumor necrosis factor inhibitors, or patients with HIV infection. In addition, there are specific entities or categories associated with EBV infection, such as lymphomatoid granulomatosis characterized by angiocentric lesions that affect the skin, lungs, or central nervous system; Fibrin-associated DLBCL in patients with chronic lymph node-confined inflammation or EBV+ intravascular proliferations. CHL should also be considered due to its variable association with EBV infection. Finally, the PTPL should be considered. In all these cases, performing a broad immunohistochemical panel is important to rule out differential diagnoses, and in the case of negative LMP1 by immunohistochemistry, perform EBER.
Regarding treatment, 3 of 6 patients initially received a chemotherapy regimen for CHL (ABVD), and at the time of making the correct diagnosis, they were administered R-CHOP (1 patient died due to disease progression); 1 case received R-ICE regimen, supplemented with whole- body radiotherapy secondary to disease progression; case 6 still does not receive treatment due to being a recently diagnosed case. A recent study by Witte et al., mentions that standard R-CHOP immunochemotherapy induces a non-inferior response in EBV+ DLBCL-NOS, compared to DLBCL-NOS, while therapeutic intensification in the first- line setting (eg, high-dose chemotherapy followed by ASCT) seems expendable. The authors’ concluding observations suggest a prognostic advantage in R-CHOP-treated patients with borderline performance status/age [20]. The work of Witte et al., was part of several studies that initiated therapies targeting CD30+ lymphomas (eg, brentuximab vedotin), seeking to counteract this adverse prognostic effect in the small group of EBV+ DLBCL-NOS. This notion is further supported by the fact that beyond its mere CD30 positivity by immunohistochemistry, the strong activation of NF-kappa in classical Hodgkin lymphoma is widely believed to promote the therapeutic efficacy of brentuximab vedotin. In our series, two patients started therapy with brentuximab vedotin, having a good therapeutic response, with stable disease and decreased symptoms and nodal and organic infiltration. Despite the poor prognosis of this entity being mentioned in the literature (1), only 2 of the patients in our series died (1 adult and 1 child), this was due to disease progression, and because anti-CD20 therapy started late.
Conclusion
EBV+ DLBCL-NOS is a rare lymphoma with rapid clinical progression and infiltration of lymph nodes and extranodal organs. In our opinion, at least in Mexico, this type of case is underdiagnosed, since patients receive chemotherapy regimens for other types of lymphoma, causing disease progression, and changing the chemotherapy regimen only when the correct diagnosis is made. We also believe that suspicion in pediatric and adolescent patients is practically null, especially because, despite recent research already mentioned in this manuscript, due to the frequency and low suspicion in children, cases are diagnosed as B-cell. lymphoma, unclassifiable, with intermediate characteristics between DLBCL and CHL, also known as gray zone lymphoma, although in this type of lymphoma the overlap of histological and immunohistochemical characteristics are different, and the expression of EBV, either by LMP1 or EBER must possibly raise suspicion of EBV+ LDBCL-NOS; also It is also easier to think of a CHL with transformation to a DLBCL. Consider that it is important to adequately explore the clinical context, and especially the follow-up of patients, since these occasions are likely to lead to resistance to treatment. We also believe that suspicion in pediatric and adolescent patients is practically null. It is recommended in all patients with HRS-like neoplastic cells, centroblast or immunoblast type, with a polymorphic or monomorphic pattern, to perform B-cell markers (CD20, PAX5), and to detect EBV using immunohistochemistry or in situ hybridization. We believe that the patients in our series expressed LMP1 by immunohistochemistry because the prevalence of EBV infection in the latent phase is high in our country. The realization of CD30 is also suggested, with the possibility of receiving targeted soft therapy for this marker. Finally, a deeper understanding of this entity would avoid prolonged treatment and high morbidity and mortality due to disease progression.
References
- Nakamura S. EBV-positive diffuse large B-cell lymphoma, 4th Edition. 2017.
- Ok CY, Papathomas TG, Medeiros LJ, Young KH. EBV-positive diffuse large B-cell lymphoma of the elderly. Blood 2013;122:328–40.
[Crossref] [Google scholar] [PubMed].
- Monabati A, Vahedi A, Safaei A, Noori S, Mokhtari M, Vahedi L, et al. Epstein-Barr virus-positive diffuse large B-cell lymphoma: Is it different between over and under 50 years of age?. Asian Pac J Cancer Prev 2016;17:2285–89.
[Crossref] [Google scholar] [PubMed].
- Oyama T, Ichimura K, Suzuki R, Suzumiya J, Ohshima K, Yatabe Y, et al. Senile EBV+ B-Cell Lymphoproliferative Disorders A Clinicopathologic Study of 22 Patients. Am J Surg Pathol 2003.27: 16-26.
[Crossref] [Google scholar] [PubMed].
- Nicolae A, Pittaluga S, Abdullah S, Steinberg SM, Pham TA, Davies-Hill T, et al. EBV-positive large B-cell lymphomas in young patients: A nodal lymphoma with evidence for a tolerogenic immune environment. Blood 2015;126:863–72.
[Crossref] [Google scholar] [PubMed].
- Zhou Y, Xu Z, Lin W, Duan Y, Lu C, Liu W, et al. Comprehensive Genomic Profiling of EBV-Positive Diffuse Large B-cell Lymphoma and the Expression and Clinicopathological Correlations of Some Related Genes. Front Oncol 2019;9:683.
[Crossref] [Google scholar] [PubMed].
- Wang E, Papavassiliou P, Sebastian S. A malignant lymphoma with histological features and immunophenotypic profile intermediate between EBV-positive diffuse large B-cell lymphoma and EBV-positive classical Hodgkin lymphoma in a 67-year-old female: A “gray”. zone” lymphoma associated with Epstein-Barr virus in the elderly. Pathol Res Pract 2012;208:363–67.
[Crossref] [Google scholar] [PubMed].
- Castillo JJ, Beltran BE, Miranda RN, Young KH, Chavez JC, Sotomayor EM. EBV-positive diffuse large B-cell lymphoma, not otherwise specified: 2018 update on diagnosis, risk-stratification and management. American Journal of Hematology 2018;93:953–62.
[Crossref] [Google scholar] [PubMed].
- Chabay P. Advances in the pathogenesis of EBV-associated diffuse large B cell lymphoma. Cancers 2021;13:2717.
[Crossref] [Google scholar] [PubMed].
- Bourbon E, Maucort-Boulch D, Fontaine J, Mauduit C, Sesques P, Safar V, et al. Clinicopathological features and survival in EBV-positive diffuse large B-cell lymphoma not otherwise specified. Blood Adv 2021;5:227–39.
[Crossref] [Google scholar] [PubMed].
- Cohen M, Narbaitz M, Metrebian F, de Matteo E, Preciado M V, Chabay PA. Epstein-Barr virus-positive diffuse large B-cell lymphoma association is not only restricted to elderly patients. Int J Cancer 2014;135:2816–24.
[Crossref] [Google scholar] [PubMed].
- Beltran BE, Castro D, Paredes S, Miranda RN, Castillo JJ. EBV-positive diffuse large B-cell lymphoma, not otherwise specified: 2020 update on diagnosis, risk-stratification and management. Am J Hematol 2020; 95: 435–45.
[Crossref] [Google scholar] [PubMed].
- Hwang J, Suh CH, Kim KW, Kim HS, Armand P, Huang RY, et al. The incidence of epstein-barr virus-positive diffuses large b-cell lymphoma: A systematic review and meta-analysis. Cancers 2021; 13: 1785.
[Crossref] [Google scholar] [PubMed].
- IARC Working Group on the Evaluation of Carcinogenic Risk to Humans Biological Agents—Epstein-Barr Virus. In: IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. ISBN 9789283213192. 2012; 45–88.
- Shannon-Lowe C, Rickinson A. The Global Landscape of EBV-Associated Tumors. Front Oncol 2019; 9: 713.
[Crossref] [Google scholar] [PubMed].
- Biggi AFB, de Oliveira DE. The Epstein - Barr virus Hacks Immune Checkpoints: Evidence and Consequences for Lymphoproliferative Disorders and Cancers. Biomolecules 2022; 12: 397.
[Crossref] [Google scholar] [PubMed].
- Crombie JL, LaCasce AS. Epstein Barr virus associated B-cell lymphomas and iatrogenic lymphoproliferative disorders. Front Oncol 2019; 9: 109.
[Crossref] [Google scholar] [PubMed].
- Huang S, Yasuda T. Pathologically Relevant Mouse Models for Epstein–Barr Virus–Associated B Cell Lymphoma. Front Immunol 202; 12: 639844.
[Crossref] [Google scholar] [PubMed].
- Wang Y, Wang C, Cai X, Mou C, Cui X, Zhang Y, et al. IL-21 Stimulates the expression and activation of cell cycle regulators and promotes cell proliferation in EBV-positive diffuse large B cell lymphoma. Sci Rep 2020; 10: 12326.
[Crossref] [Google scholar] [PubMed].
- Witte HM, Merz H, Biersack H, Bernard V, Riecke A, Gebauer J, et al. Impact of treatment variability and clinicopathological characteristics on survival in patients with Epstein-Barr-Virus positive diffuse large B cell lymphoma. Br J Haematol 2020; 189: 257–68.
[Crossref] [Google scholar] [PubMed].
Copyright: © 2022 The Authors. This is an open access article under the terms of the Creative Commons Attribution NonCommercial ShareAlike 4.0 (https://creativecommons.org/licenses/by-nc-sa/4.0/). This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.