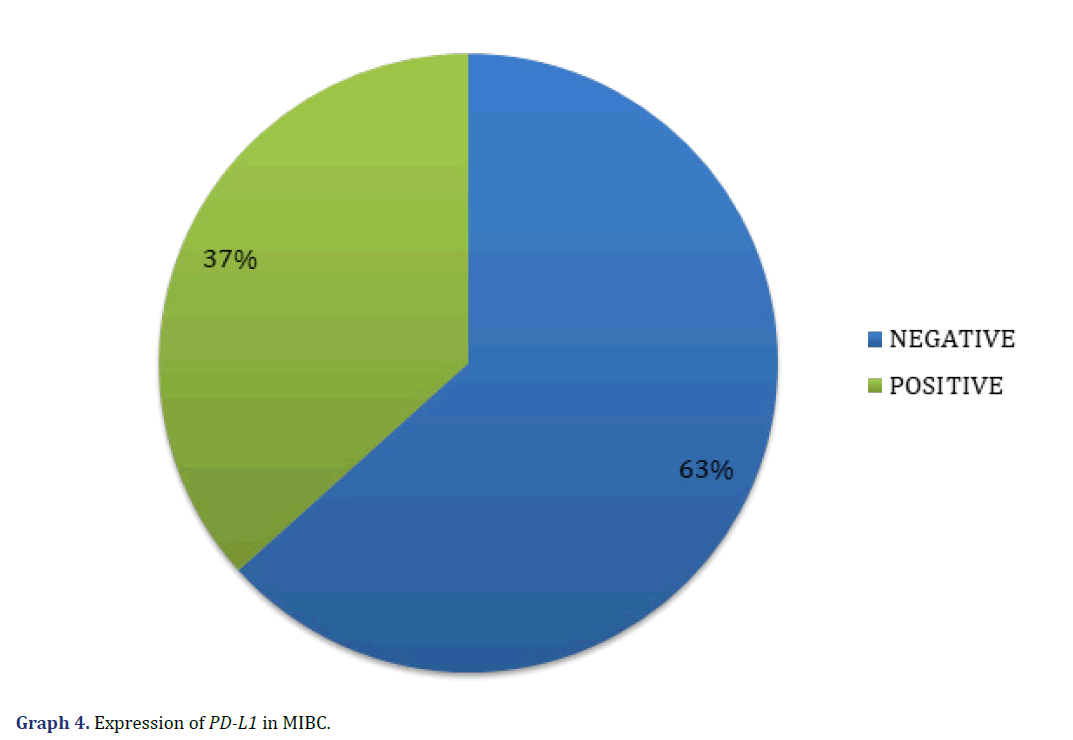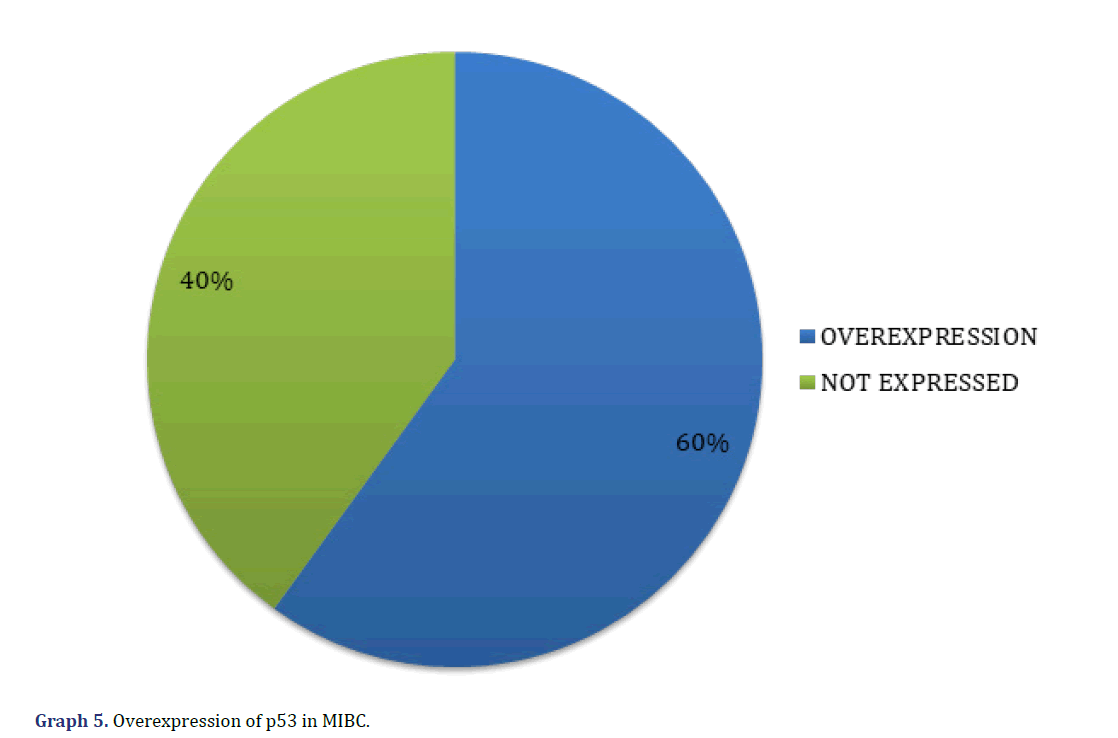Research Article - Journal of Interdisciplinary Histopathology (2022)
Expression of PDL1 and P53 in Muscle-Invasive Bladder Carcinoma: A New Alternative for Neoadjuvant Treatment in Urothelial Cancer
Karen Sarahí Vázquez-Rodrígue1, Lázaro A Ramírez-Balderrama1, Luis Jonathan Bueno-Rosario2, Saulo Mendoza-Ramírez3, Marco Antonio Olvera-Olvera1 and Mario Murguia-Perez1*2Department of Surgical Pathology, Hospital Regional de Alta Especialidad del Bajío, Guanajuato, Mexico
3Department of Surgical Pathology, ABC Observatory Medical Center, Mexico City, Mexico
Mario Murguia-Perez, Department of Surgical Pathology, Mexican Institute of Social Security Leon, Guanajuato, Mexico, Email: drmariopatologia@gmail.com
Received: 26-Nov-2022, Manuscript No. EJMJIH-22-78909; Editor assigned: 28-Nov-2022, Pre QC No. EJMJIH-22-78909 (PQ); Reviewed: 12-Dec-2022, QC No. EJMJIH-22-78909; Revised: 19-Dec-2022, Manuscript No. EJMJIH-22-78909 (R); Published: 26-Dec-2022
Abstract
Invasive Urothelial Carcinoma (IUC) is the tenth most common malignancy worldwide. Its treatment depends on the clinical stage in which it is found and based on this; there are various therapeutic options to use. Currently, immunotherapy plays a key role in the treatment of several malignant tumors, including IUC. Our work identified the expression of PD-L1 in Muscle-Invasive Bladder Carcinoma (MIBC), a field little explored at present, using a validated clone of PD-L1 (28-8, BioSB®), finding CPS>5% of PD-L1 in 35% (11/30 specimens) of MIBC cases; in addition, there was a correlation between the expression of PD-L1 with the presence of mononuclear infiltrate (p=0.002). Overexpression of p53 was also identified (60%, 18/30 specimens), but no statistical significance was found in the correlation with various variables evaluated, although we found a close relationship with muscle invasion (p=0.077). Due to the performance of multiple clinical trials where the expression of PD-L1 in MIBC is studied, we consider it important to carry it out in patients with muscle invasion, even though there is currently no therapeutic indication for neoadjuvant immunotherapy, hoping soon the approval of it.
Keywords
Invasive urothelial carcinoma; Monoclonal antibodies; Immunohistochemistry; PDL1; p53
Introduction
Infiltrating Urothelial Carcinoma (IUC) is the most common malignant neoplasm of the urinary tract, being the tenth most frequent worldwide, with a 3-4:1 ratio between men and women. The average age of diagnosis is between 65 and 70 years; mortality is different between both genders, estimating 2-10 deaths per 100,000 men per year and 0.5 to 4 deaths per 100,000 women per year [1]. About 75% of urothelial carcinomas are non-invasive. IUCs account for 20% to 40% of cases, and the standard of care is radical cystectomy with or without neoadjuvant chemotherapy or concurrent chemoradiation as an option to preserve the bladder. However, even after treatment, more than 50% of patients show recurrence and most of them die from metastatic disease within 3 years of diagnosis [2,3]. Bacillus Calmette-Guerin (BCG), chemotherapy, or radiotherapy is combined in the procedure of transurethral resection of bladder tumor to reduce the rate of recurrence; however, BCG increases the immune response in some patients, while chemotherapy and radiotherapy have been associated with painful side effects [4].
Before PD-L1/PD-1 checkpoint inhibitors, systemic chemotherapy with cisplatin-based systems was the standard of care, giving a median survival of 1 year. For patients with platinum-refractory disease median survival was 6-9 months, however, patients with 30%-50% metastatic disease were not candidates for cisplatin due to treatment comorbidities [2].
Immunotherapeutic agents targeting Programmed Death receptor 1 (PD-1) and Programmed Death Ligand 1 (PD-L1) are currently therapeutic alternatives in IUC. As in other tumors, high rates of objective response have been observed in patients with high expression of PD-L1. The medications used are approved by the US Food and Drug Administration (FDA), along with a clinical trial. Recently, first-line use of atezolizumab and pembrolizumab in platinum- ineligible patients has been restricted to patients with PD-L1-positive tumors due to the high mortality rates of PD-L1-negative patients receiving checkpoint inhibitors in chemotherapy sites [5]. 50% of IUC acquire p53 inactivation mechanism to avoid apoptosis 20% of bladder carcinomas are caused by mutation of the TP53 gene [4]. P53 occurs as wild- type and mutant isoforms. The wild-type form of p53 allows DNA from damaged cells to enter the G1/G0 arrest process and repair DNA before entering the synthesis phase (S), carried out by: I) Activation of DNA repair proteins, II) cell growth arrest at the G1/S checkpoint by identifying DNA damage, and II) inducing apoptosis if cell damage is irreparable [6]. Among other studies, Hodgson and colleagues have shown abnormal expression of p53 (nuclear staining greater than 50%) is useful for the prognosis of urothelial carcinoma; Similarly, Sjödahl et al. demonstrated that changes in p53 expression are associated with a more aggressive molecular subtype of urothelial carcinoma and that it can progress and/or be found in more advanced stages [7-9].
Having these therapeutic alternatives, especially the inhibition of PD-1 and PD-L1, it is important to carry out immunolabeling against said ligand, to determine if patients are candidates for immunological therapy. It is also important to determine the expression of p53 because of its aggressive behaviour. In Latin America, it is not performed routinely due to the high costs involved in carrying out said immunolabeling, the low availability in hospital centres, and the fact that these monoclonal antibodies are not authorized by the health systems. In addition, there are specific therapeutic indications in patients with positivity for PD-L1 in metastatic UC, in resistance to platinum-based chemotherapy, and in high-risk non-muscle invasive UC (nMIBC). However, there are very few studies that mention the expression of PDL1 and p53 in muscle-invasive CU (MIBC), which is the basis of our work.
Materials and Methods
An observational, cross-sectional, and retrospective study. The results of the pathology laboratory archive of UMAE Hospital de Especialidades No. 1, Centro Médico Nacional Bajío, Instituto Mexicano del Seguro Social (IMSS), were reviewed in a period that spanned from January 2019 to December 2021. All reports were searched with the diagnosis of invasive or infiltrating bladder carcinoma, subsequently, the slides and paraffin blocks were collected for review. The histological type was confirmed, as well as the invasion of detrusor muscle (MIBC); the presence or absence of mononuclear immune cells (lymphocytes, macrophages, multinucleated giant cells, plasma cells) associated with the tumor was determined, and a score was added according to the presence or absence of said component, with 0 being the absence of Immune Cells (IC), 1 mild inflammatory infiltrate, 2 moderate and 3 intense. In each case, the corresponding paraffin block with the presence of invasion of the muscle layer or lamina propria was identified. Immunohistochemistry was performed by automated technique with Pathcom Slide Stainer SSI System equipment, using PD-L1 (clone 28-8, BioSB®) and p53 (clone ZR153, Zeta®) antibodies. The interpretation of PD-L1 was performed using the Combined Positive Score (CPS), considering the positive case with membranous expression in 5% of tumor cells and immune cells; p53 was considered positive or overexpressed with nuclear expression in 80% of tumor cells. Finally, clinical parameters were collected from the patient’s electronic records (age, sex, affected anatomical site). Descriptive statistics were performed to determine percentages and frequencies, and for the correlation analysis of variables, we used Pearson’s correlation analysis, considering statistical significance as p ≤ 0.05. Statistical analysis was carried out in IBM® SPSS® Statistics 21 software.
Results
In the estimated time, 45 cases diagnosed as IUC were identified, of which 14 cases were excluded: 8 of them presented 2 or more histopathology reports corresponding to the same patient, 5 cases were eliminated because the histopathological review concluded that there was no infiltration of detrusor muscle and 1 corresponded to a urothelial carcinoma of the renal pelvis; leaving a total of 31 cases of MIBC for the study.
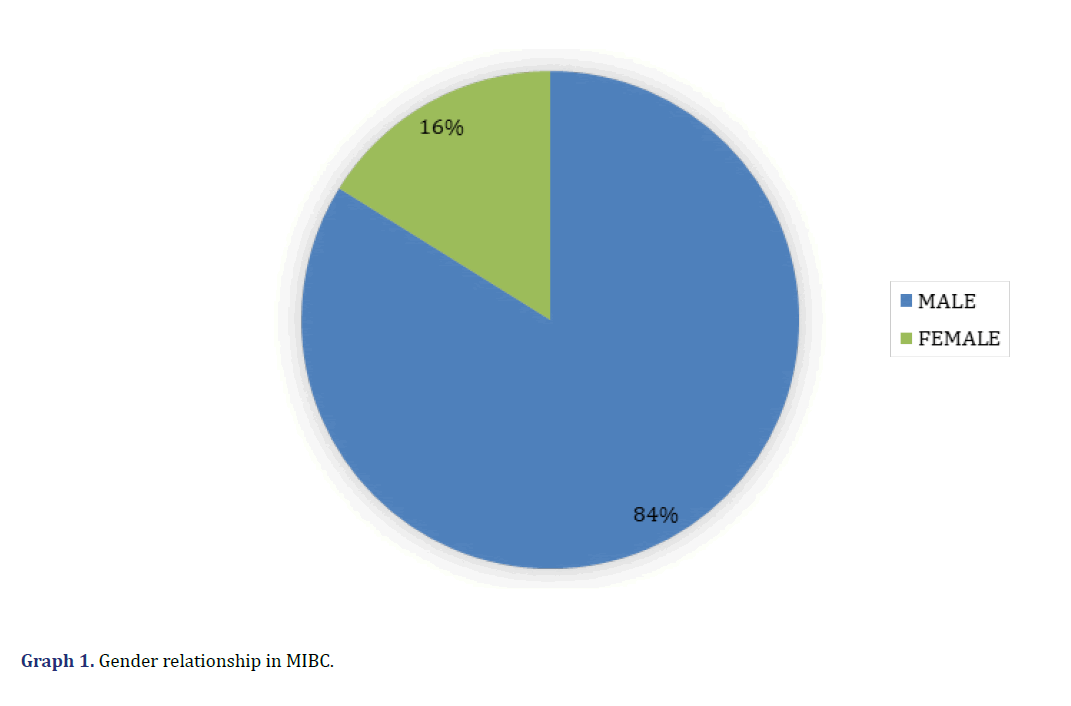
Clinically, 5 cases corresponded to women (16%) and 2 to men (84%) (Graph 1), with an age range between 41 and 90 years (mean 66.3). All cases presented haematuria at the time of the initial evaluation. In none of the cases evaluated was the affected bladder anatomical area mentioned in the electronic records.
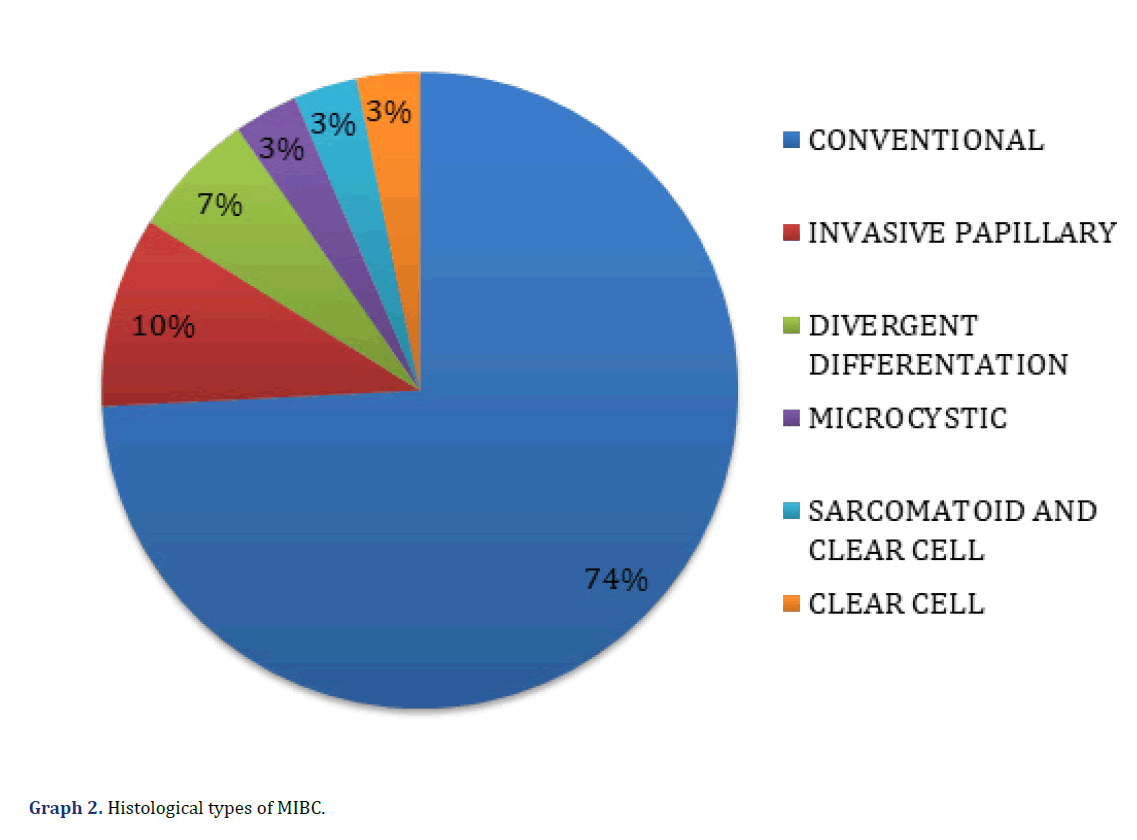
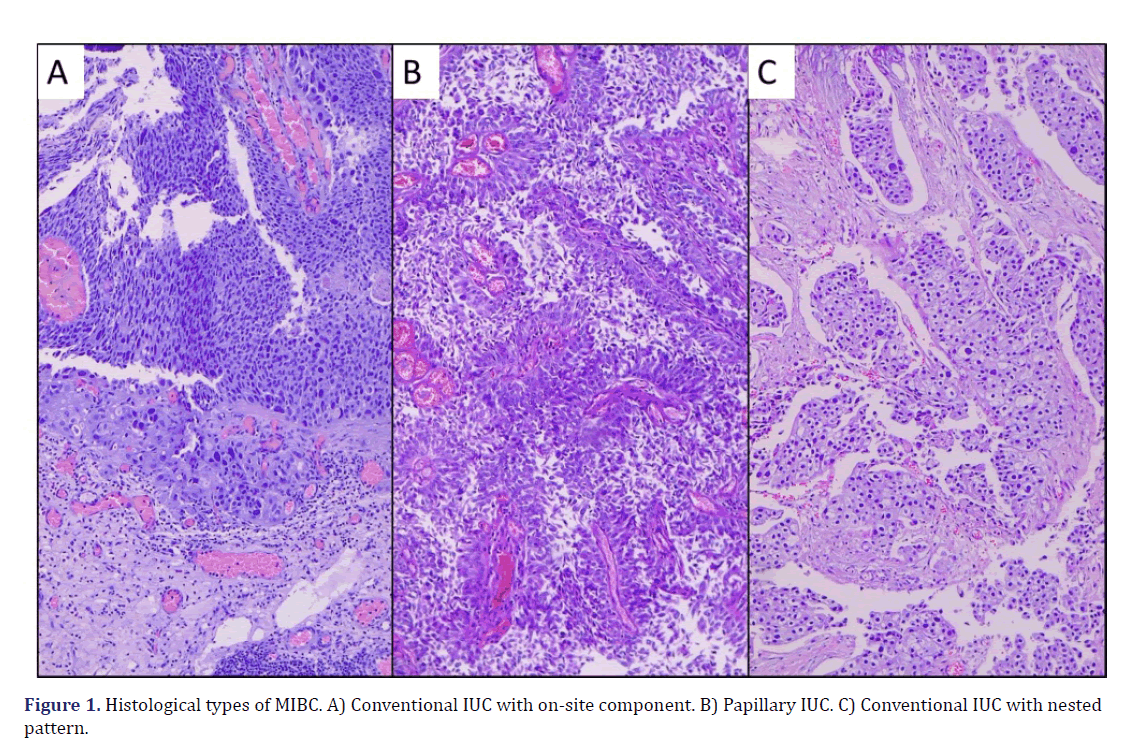
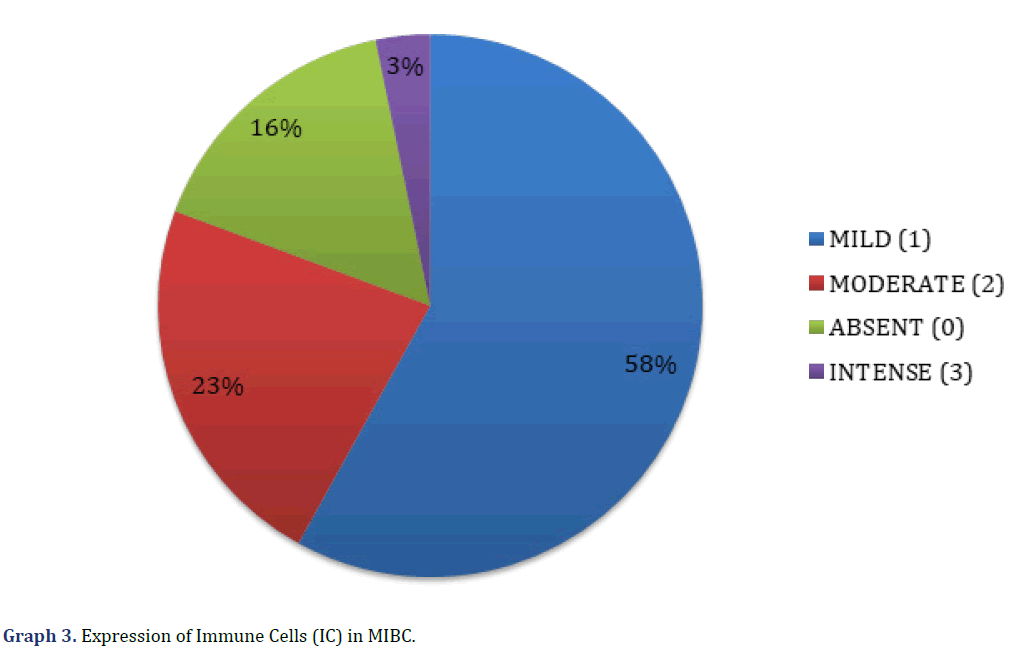
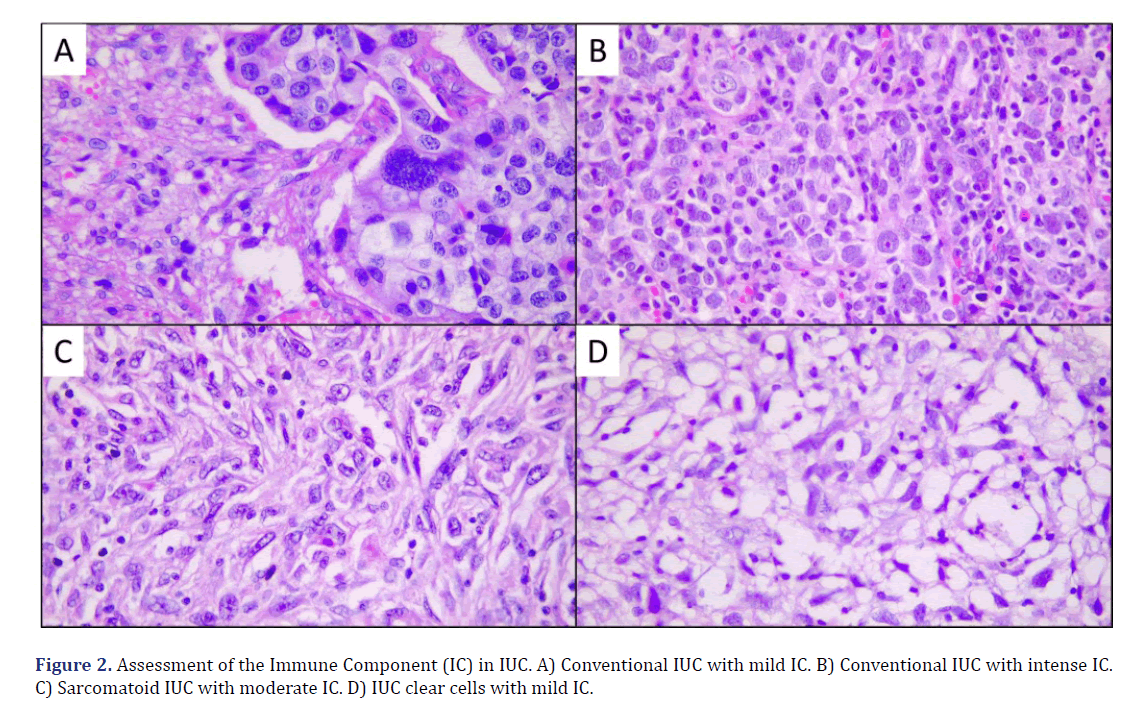
The histological types of MIBC observed in the microscopic evaluation were the conventional type (23 cases, 74%), papillary (3 cases, 10%), divergent histology (2 cases, 7%), clear cell (1 case, 3%), microcystic (1 case, 3%) and one case was mixed, with a sarcomatoid pattern and clear cell (3%) (Graph 2) (Figure 1). The presence of peritumoral IC was found in 26 cases (84%), with score 1 (mild IC 18 cases, 58%), score 2 (moderate IC, 7 cases, 23%), and score 3 (intense IC, 1 case, 3%); in 5 cases (16%) no IC (score 0) was observed (Graph 3) (Figure 2).
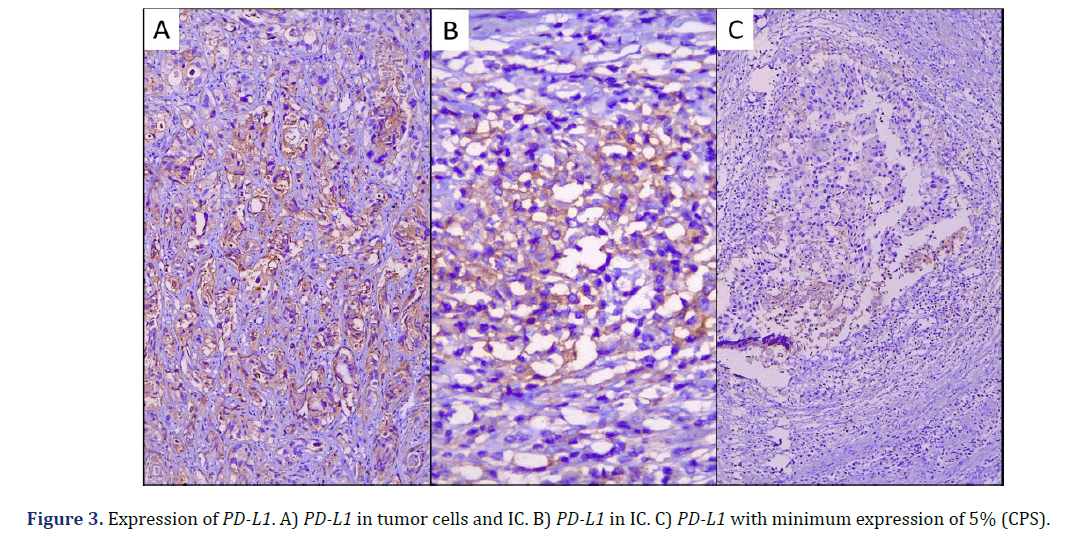
In the immunohistochemical studies, one case was not evaluated because no invasive tumor was observed in the subsequent cuts; of the remaining 30 cases, we observed PD-L1 expression with CPS > 5% in 11 cases (all positive, 37%), and 19 cases with CPS<5% (all negative, 63%) (Graph 4) (Figure 3).
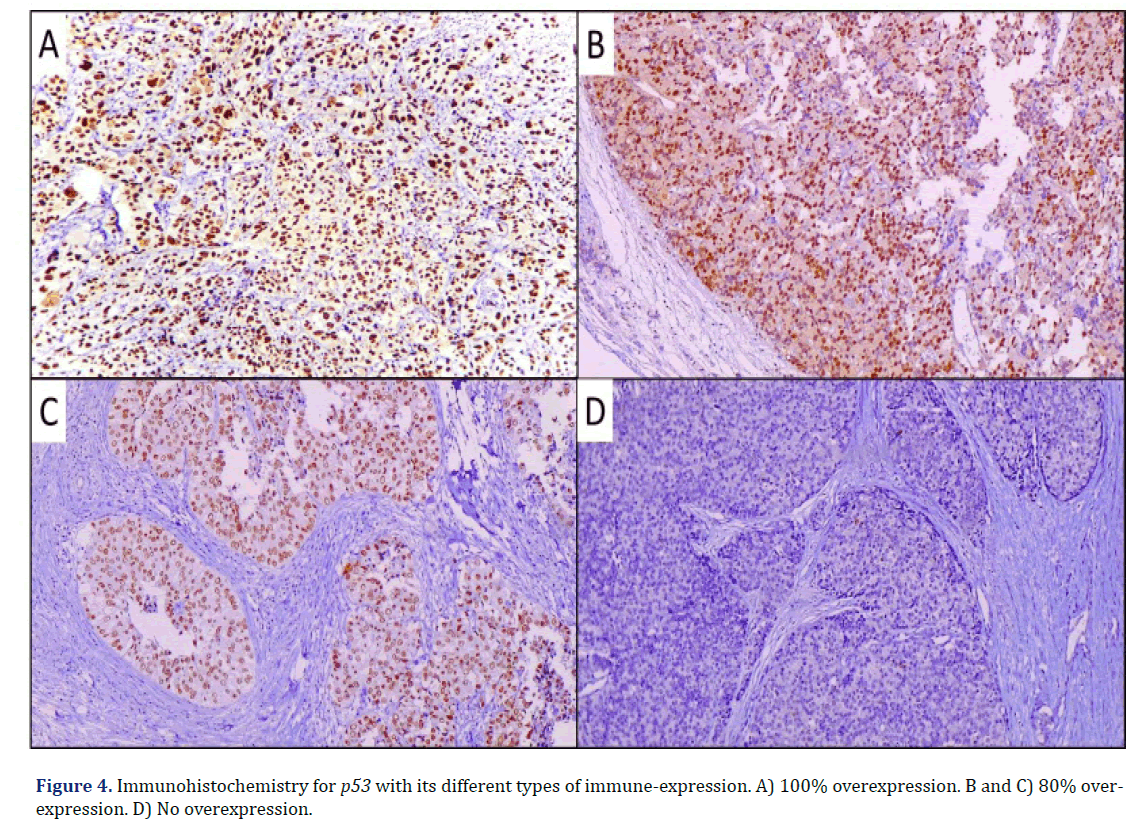
Regarding p53, we found 18 cases (60%) with p53 overexpression and 12 cases (40%) not overexpressed (Graph 5) (Figure 4).
Concerning the inferential statistical analysis, the possible correlation between every one of the variables mentioned above was sought with the Pearson method, we observed that the correlation between PD-L1 with CPS>5% and the presence of IC was statistically significant with a p=0.002 and a Pearson correlation strength of 0.53, being the only one found among the analysis of all the variables. No statistical significance was identified between p53 overexpression and the degree of muscle invasion (p=0.077), as well as between PD-L1 expression with CPS>5% and the histological variety (p=0.093). The results of the inferential statistical analysis are summarized in (Table 1).
| Age | Gender | Histologic Type | Immune cells | p53 overexpression |
PD-L1 positivity |
Detrusor muscle invasion | ||
|---|---|---|---|---|---|---|---|---|
| Age | Pearson correlation | 1 | -0.021 | 0.054 | .1 00 | 0.086 | 0.177 | 0.18 |
| Sig. (bilateral) | 0.911 | 0.793 | 0.599 | 0.652 | 0.351 | 0.342 | ||
| N | 30 | 30 | 26 | 30 | 30 | 30 | 30 | |
| Gender | Pearson correlation | -0.021 | 1 | -0.191 | 0.166 | -0.103 | 0.031 | -0.12 |
| Sig. (bilateral) | 0.911 | 350 | 0.38 | 334 | 0.871 | 0.59 | ||
| N | 30 | 30 | 26 | 30 | 30 | 30 | 30 | |
| Histologic type | Pearson correlation | 0.054 | -0.191 | 1 | -69 | 0.138 | 0.337 | |
| Sig. (bilateral) | 0.793 | 0.35 | 738 | 502 | 93 | 0 | ||
| N | 26 | 26 | 26 | 26 | 26 | 26 | 26 | |
| Immune cells | Pearson correlation | 0.100 | 0.166 | -0.069 | 1 | 0.152 | 0.533 | -0.05 |
| Sig. (bilateral) | 0.599 | 0.38 | 738 | 0. 4 24 | 0. 002 | 0.795 | ||
| N | 30 | 30 | 26 | 30 | 30 | 30 | 30 | |
| p53 overexpression | Pearson correlation | 0.086 | -0.183 | 0.1 38 | 0.152 | 1 | 0.339 | -327 |
| Sig. (bilateral) | 0.652 | 0.334 | 502 | 0.424 | 67 | 77 | ||
| N | 30 | 30 | 26 | 30 | 30 | 30 | 30 | |
| PD-L1 positivity | Pearson correlation | 0.177 | 0.031 | 337 | 533 | 339 | 1 | -203 |
| Sig. (bilateral) | 0.351 | 0.871 | 93 | 0.002 | 67 | 0.281 | ||
| N | 30 | 30 | 26 | 30 | 30 | 30 | 30 | |
Note: The correlation is significant at the level 0.01 (bilateral). |
||||||||
Discussion
Urothelial Carcinoma (UC) is a well-recognized immunogenic and immune-sensitive tumor; Intravesical BCG therapy has been used for decades for non-muscle invasive urothelial carcinomas [10] as well as radiotherapy and chemotherapy; however, patients with aggressive carcinomas benefit little from these therapies [11]. In recent genomic studies, it has been shown that urothelial carcinoma has the fourth highest load of mutations, which as a consequence can trigger an immune response; furthermore, tumor-associated IC may be related to clinical outcomes of urothelial carcinoma [10]. Tumor cells can suppress the activity of the immune system by overproducing immunosuppressive factors or by exposing the surface ligand that inhibits lymphocytes, activating the receptor for Programmed Death-1 (PD-1) and cytotoxic T-lymphocyte associated protein 4, these are known as “immune checkpoints” [12]. Check Point Inhibitors (CPIs) are monoclonal antibodies developed to attack the inhibitory pathways of the immune system [12].
Cell surface Programmed Death Ligand-1 (PD-L1) has been investigated as a biomarker of responsiveness to Programmed Death blockade (PD-1) or combination immunotherapy in cancer [13] which is crucial for the modulation of the immune system to reduce collateral tissue damage from the inflammatory response to infectious microorganisms in peripheral tissues [14]. PD-1 (encoded by the CD279 gene) [15] is a cell surface receptor that belongs to the immunoglobulin superfamily and is also a member of the extended CD28/CTLA-4 family. The extracellular region of PD-1 is 28% identical to CTLA-4, another immune checkpoint molecule [14]. PD-1 mediates regulatory functions through interaction with two ligands, PDL1 and PD-L2. Many types of immune cells express PD-L1, including T cells, B cells [14-16] macrophages, Dendritic Cells (DCs), epithelial cells, stromal cells such as fibroblasts, endothelial cells, and tumor cells [14-16]. PD-L2 has a high affinity for PD-1 and is expressed by DCs, macrophages, B cells, Th2 cells, and some lung epithelial cells [16]. PD-1 is a costimulatory molecule, and once activated by its ligands (PD- L), PD-1 (probably along with the other cooperative pathways) inhibits kinases involved in T-cell activation via phosphatase (SHP). When T cells are exposed to chronic antigenic stimulation, such as chronic viral infection or cancer, persistently high levels of PD-1 expression are induced and lead to T cell exhaustion or anergy [14]. Under physiological conditions, the PD-1/PD-L axis is crucial for the regulation of immune responses to minimize collateral tissue damage and maintain homeostasis in the body through the selective recognition and elimination of pathogens and abnormal cells [14,15]; however, hyper activation of uncontrolled T cells can also attack normal cells [15] and in neoplastic disease, since PD-Ls are aberrantly expressed in tumor cells and/or Tumor-Associated Inflammatory cells (TAI) help tumor cells evade the host’s immune system and promote tumor growth [14].
The response rate of urothelial carcinoma to CPIs in metastatic patients is 15-21%. Currently, the predictive biomarkers that have been widely studied and used are the expression of PD-1, PD-L1, the inflammatory microenvironment, and mutational load [10]. The immuno-oncological agents PD1 and PD-L1 have been studied as first and second-line treatments in metastatic urothelial carcinoma. Pembrolizumab and atezolizumab have been approved by the Food and Drug Administration (FDA) as first-line treatments for patients who are not candidates for cisplatin with stage IV UC and high expression of PD-L1 in the tumor [17].
Tumor cells interact with their microenvironment to escape immune attacks. Based on chronic inflammation and PD-L1 expression, the microenvironment can be divided into “immunogenic” (rich in immune cells) or “non-immunogenic” (lacking in immune cells), these two categories have been suggested in multiple studies as an indicator for response to immunotherapy, being immunogenic that presents a better response to immunotherapy [10].
CPI therapies have shown promising efficacy in carcinomas, including urothelial carcinoma. Detection of PD-L1 expression has been adapted in complementary tests to predict response to CPIs. In 2020, Li et al. showed that the PD-L1 score in a tumor microenvironment was significantly correlated to chronic inflammation (CD3+cells and CD8+ cells), consistent with the idea that immune tolerance is associated with an immunogenic microenvironment [10]. In our population, we also demonstrated the correlation between PD-L1 positivity (CPS>5%) and IC. In 2019, Ketan Ghate et al. conducted a study in which they concluded that PD-L1 expression is associated with a better response rate with CPI anti-PD-L1; however, the results of their meta-analysis did not show any association between PD-L1 expression and overall survival and concluded that the development of biomarkers should become a priority to identify those patients with a greater possibility of response [18]. In contrast to these results, in 2021 Jing Xu et al. conducted a study in which they found that PD-L1 methylation is an independent prognostic factor in urothelial carcinoma; its hypermethylation indicates worse survival for patients with urothelial carcinoma; however, it indicates the need for further studies focused on the response to immunotherapy [19].
Aberrant expression of p53 has been found in up to 50% of patients with high-grade UC [11], playing an important role in tumor growth and progression [20], which has been associated with a worse prognosis [21]. P53 is an essential transcription factor for genes in the damage response as an inhibitor of CDK21, growth arrest, DNA damage, and BAX protein. After activation of p53, they will initiate the expression of certain genes (gene p21 and GADD45) that will act by stopping the cell cycle and repairing the damaged DNA. If the damage is irreparable, p53 will activate the transcription of the BAX gene by binding to BCL-2 and initiating apoptosis. P53 function can be restored by various means, for example, delivery of wild-type p53 by viruses, delivery of p53 peptide by nonviral methods, and manipulation of p53 regulators [11]; Despite this, in 2020, Ziaran et al. did not find a significant association with the specific survival of this carcinoma with only the expression of p53 [21]. Most IUCs present alterations in p53, as well as presenting a higher mutational load. However, in our study, we did not find statistical significance between p53 overexpression and invasion of muscle layers; with the data, we found a trend that, although it was not statistically significant, in a larger study and with stricter control of variables, this trend may be confirmed as significant.
Pathologic staging of the tumor is primarily based on the extent of invasion to the deeper layers of the bladder and is divided according to superficial bladder carcinomas or MIBC. Patients with nMIBC would be at risk for recurrence and disseminated disease after initial treatment dictating the need for additional therapy. The 2016 European Association of Urologists (EAU) guidelines define different risks of progression based on tumor grade, lamina propria invasion, tumor size, and whether the tumor is recurrent or multifocal. Conservative management is preferred to potentially allow preservation of a functional bladder based on transurethral resection of bladder tumor, potentially combined with adjuvant intravesical therapy. Patients classified as low risk are usually treated with TURBT alone, plus a single perioperative dose of intravesical chemotherapy with mitomycin. Intermediate or high- risk nMIBC are usually treated with additional intravesical therapy to decrease the risk of recurrence or progression, usually with BCG therapy, although alternatives are offered due to the existing shortage of BCG [22]. In patients with MIBC, the treatment of choice is radical cystectomy, although multimodal therapy is also considered a viable alternative option. Cisplatin-based neoadjuvant chemotherapy has been shown to reduce the risk of recurrence and improve overall survival compared to surgery alone [23]. Standard treatment in patients with muscle-invasive bladder cancer includes cisplatin-based chemotherapy followed by surgical removal of the bladder, Radiation Therapy (RT), and/or concomitant chemotherapy. Cisplatin-based neoadjuvant chemotherapy before cystectomy or RT improves overall survival [24]. The current indications for the use of CPI with pembrolizumab and atezolizumab are 1) second-line treatment for chemotherapy failure in metastatic UC; 2) first-line treatment for metastatic UC in patients who are not candidates for platinum-based therapy; 3) first-line treatment in high-risk nMIBC [25]. In a study by Vidotto et al, strong evidence was found for the expression of multiple immune checkpoint genes, including PD-1, PD-L1, IDO1, TIGIT, TIM-3, TGFB1, LAG3, and others, potentially contribute to compensatory immune evasion in bladder tumors; this highlights the urgent need for biomarker discovery approaches that combine molecular subtyping, DDR gene mutation status, tumor immune environment classification, and immune checkpoint gene expression to increase the number of patients who respond to immunotherapy [26]. Although currently it has not been considered as a first or second line of treatment in MIBC, there are multiple phase I and II clinical trials that are exploring the possibility of neoadjuvant immunotherapy, either as monotherapy or combined with CT, with encouraging results, which opens the possibility of new treatment schemes for patients with MIBC [27-29].
Despite the extensive literature, and unlike in lung carcinoma, tests for PD-L1 expression in UC are not yet standardized and different methods are used by various institutions, and it is unknown whether different tests can be easily interchanged with different treatments or between indications. Furthermore, PDL1 expression is heterogeneous within tumors, between the primary tumor and the metastasis, and can come and go over time. While PD-L1 expression tests are becoming more widely available, they are opening conversations about treatment options between providers and patients [25-30]. The clones validated in various studies for the assessment of PD-L1 are 22C3 (Dako®), SP142, SP263 (Ventana®), and 28-8 (Dako®, BioSB®). However, anti-PD-L1 with clones from other commercial companies can be harmonized for use. In a study carried out at the Medical Center of the American University of Beirut, 54 histopathological specimens of radical cystectomies with MIBC were anain lyzed, and PD-L1 clone 5H1 was performed (they do not specify a commercial company), defining positivity with CPS>5%, and finding that 9% (5 patients) showed positivity for PD-L1, and of the positive cases, 4 presented metastases in iliac lymph nodes, and only 2 of these 4 specimens were positive [29]. In our study, we found that 11/31 histopathological specimens (35%) showed PD-L1 expression with a validated antibody, and it is likely that the difference between the previously cited study and ours is due to the use of the clone 5H1 and 28-8, respectively. As explained previously, the different clones can be harmonized; however, this requires studies with multiple specimens and comparison with the validated standard. In addition, we do not have the type of immunohistochemical technique used in the Mukherji study (manual or automated), while we used an automated technique, which allowed us to perfectly control the times in each of the steps of the process, and guaranteed that the same will apply to all specimens. In our hospital center, we also have another clone of PD-L1 (ZR3, Zeta®), which we have used on occasions to compare the expression results, and reported to the oncologists in the pathology reports, with an explanatory note of which one was used, the result of the validated antibody. Until now, we have not been able to harmonize the ZR3 clone, however, in other pathologies (eg, TNBC) the ZR3 clone has been used to determine the CPS and make decisions about the treatment of patients [31].
Conclusion
There is evidence of the impact of the expression of PD-L1 and the risk of recurrence in MIBC, as well as the potential use of immunotherapy as neoadjuvant therapy, therefore, given the high prevalence of PD-L1 in our population and considered a prognostic marker, should be reconsidered for use in patients with early disease. The present study shows that, although our sample was small compared to published studies, there is an expression of PD-L1 in MIBC. Although we did not find statistical significance in the correlation between the overexpression of p53 and MIBC, we did observe a trend, which should be studied with a larger sample and stricter control of variables.
Author Contributions
Conceptualization, M.M.P.; methodology, M.M.P., L.J.B.R. and L.A.R.B..; software, L.A.R.B..; validation, M.M.P., K.S.V.R. and L.A.R.B..; formal analysis, M.M.P. and L.A.R.B..; investigation, K.S.V.R..; resources, K.S.V.R. and L.A.R.B.; data curation, M.M.P., K.S.V.R. and L.A.R.B.; writing-original draft preparation, M.M.P.; writing-review and editing, M.M.P. and K.S.V.R., M.A.O.O.; visualization L.J.B.R. and S.M.R.; supervision, M.M.P. and L.A.R.B.; project administration, M.M.P.; funding acquisition, M.M.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
This study followed the Declaration of Helsinki. Following Mexican laws with less risk to the minimum. Informed consent was exempted by the Local Research and Ethics Committee of UMAE Hospital de Alta Especialidad N˚ 1 Bajío, Instituto Mexicano del Seguro Social. All experimental protocols were approved by the Local Research and Ethics Committee of UMAE Hospital de Alta Especialidad N° 1 Bajío, Instituto Mexicano del Seguro Social, with register CONBIOETICA 11 CEI 003 2018080 and register COFEPRIS 17 CI 11 020 146. The number of institutional register of this research is R-2021-1001-124.
Informed Consent Statement
Not applicable for studies not involving humans.
Data Availability Statement
Not applicable.
Acknowledgements
H.T. Josué Camarena Quiroz, for performing the histological sections and supervising the immunohistochemistry performed on the Pathcom Slide Stainer SSI System platform. Dra. Maria Virgilia Soto-Abraham for the teachings and experiences learned during the corresponding author’s time in residence in Pathology.
Conflicts of Interest
The authors declare that they have no competing interests in the elaboration of this investigation.
Orchid
Mario Murguia-Perez https://orcid.org/0000- -4260-389X
References
- Williamson SR, McKenney JK, Raspollini MR. WHO Classification of Tumours Editorial Board Urinary and male genital tumours. 5th ed. Lyon, France: Internacional Agency for Research on Cancer; 2022.
- Stenehjem DD, Tran D, Nkrumah MA, Gupta S. PD1/PDL1 inhibitors for the treatment of advanced urothelial bladder cancer. Onco Targets Ther 2018;11: 5973–5989.
[Crossref] [Google Scholar] [PubMed].
- Felsenstein KM, Theodorescu D. Precision medicine for urothelial bladder cancer: Update on tumour genomics and immunotherapy. Nat Rev Urol 2018;15: 92–111.
[Crossref] [Google Scholar] [PubMed].
- Mansor SF. Manipulation of p53 protein in bladder cancer treatment. IMJM 2021;20: 137–147.
[Crossref] [Google Scholar].
- Eckstein M, Erben P, Kriegmair MC, Worst TS, Weiß C aron, Wirtz RM, et al. Performance of the food and drug administration / EMA-approved programmed cell death ligand-1 assays in urothelial carcinoma with emphasis on therapy stratification for first-line use of atezolizumab and pembrolizumab. Eur J Cancer 2019;106: 234–243.
[Crossref] [Google Scholar] [PubMed].
- Zheng L, Zhu Y, Lei L, Sun W, Cheng G, Yang S. Significant expression of CHK1 and p53 in bladder urothelial carcinoma as potential therapeutic targets and prognosis. Oncol Lett 2018;15: 568–574.
[Crossref] [Google Scholar] [PubMed].
- Ziaran S, Harsanyi S, Bevizova K, Novakova ZV, Trebaticky B, Bujdak P, et al. on m er ci us e on on m er ci al. 2020;64.
- Chen L, Liu Y, Zhang Q, Zhang M, Han X, Li Q, et al. p53 / PCDH17 / beclin-1 proteins as prognostic predictors for urinary bladder cancer. J Cancer 2019;10: 6207-6216.
[Crossref] [Google Scholar] [PubMed].
- Zheng L, Zhu Y, Lei L, Sun W, Cheng G, Yang S. Significant expression of CHK1 and p53 in bladder urothelial carcinoma as potential therapeutic targets and prognosis. Oncol Lett 2018;15: 568–574.
[Crossref] [Google Scholar] [PubMed].
- Li H, Zhang Q, Shuman L, Kaag M, Raman JD, Merrill S, et al. Evaluation of PD-L1 and other immune markers in bladder urothelial carcinoma stratified by histologic variants and molecular subtypes. Sci Rep 2020;10: 1439.
[Crossref] [Google Scholar] [PubMed].
- Mansor FS. Manipulation of p53 protein in bladder cancer treatment. IMJM 2021.
[Crossref] [Google Scholar].
- Pierantoni F, Maruzzo M, Gardi M, Bezzon E, Gardiman MP, Porreca A, et al. Immunotherapy and urothelial carcinoma: An overview and future prospectives. Crit Rev Oncol 2019;143: 46–55.
[Crossref] [Google Scholar] [PubMed].
- Daassi D, Mahoney KM, Freeman GJ. The importance of exosomal PDL1 in tumour immune evasion. Nat Rev Immunol 2020;20: 209–215.
[Crossref] [Google Scholar] [PubMed].
- Inaguma S, Wang Z, Lasota J, Sarlomo-Rikala M, McCue PA, Ikeda H, et al. Comprehensive immunohistochemical study of programmed cell death ligand 1 (PD-L1): Analysis in 5536 cases revealed consistent expression in trophoblastic tumors. Am J Surg Pathol 2016;40: 1133–1142.
[Crossref] [Google Scholar] [PubMed].
- Cha JH, Chan LC, Li CW, Hsu JL, Hung MC. Mechanisms controlling PD-L1 expression in cancer. Mol Cell 2019;76: 359–370.
[Crossref] [Google Scholar] [PubMed].
- Nimmagadda S. Quantifying PD-L1 expression to monitor immune checkpoint therapy: Opportunities and challenges. Cancers (Basel) 2020;12: 1–26.
[Crossref] [Google Scholar] [PubMed].
- Lopez-Beltran A, López-Rios F, Montironi R, Wildsmith S, Eckstein M. Immune checkpoint inhibitors in urothelial carcinoma: Recommendations for practical approaches to PD-L1 and other potential predictive biomarker testing. Cancers (Basel) 2021;13: 1424.
[Crossref] [Google Scholar] [PubMed].
- Ghate K, Amir E, Kuksis M, Hernandez-Barajas D, Rodriguez-Romo L, Booth CM, et al. PD-L1 expression and clinical outcomes in patients with advanced urothelial carcinoma treated with checkpoint inhibitors: A meta-analysis. Cancer Treat Rev 2019;76: 51–56.
[Crossref] [Google Scholar] [PubMed].
- Xu J, Wei L, Liu H, Lei Y, Zhu Y, Liang C, et al. CD274 (PD-L1) Methylation is an independent predictor for bladder cancer patients’ survival. Cancer Invest 2022;40: 228–233.
[Crossref] [Google Scholar] [PubMed].
- Blinova E, Samishina E, Deryabina O, Blinov D, Roshchin D, Shich E, et al. Expression of p53 protein associates with anti-PD-L1 treatment response on human-derived xenograft model of GATA3/CR5/6-negative recurrent nonmuscular invasive bladder urothelial carcinoma. Int J Mol Sci 2021;22: 9856.
[Crossref] [Google Scholar] [PubMed].
- Ziaran S, Harsanyi S, Bevizova K, Novakova ZV, Trebaticky B, Bujdak P, et al. Expression of E-cadherin, Ki-67, and p53 in urinary bladder cancer in relation to progression, survival, and recurrence. Eur J Histochem 2020;64: 3098.
[Crossref] [Google Scholar] [PubMed].
- Taylor J, Becher E, Steinberg GD. Update on the guideline of guidelines: Non-muscle- invasive bladder cancer. BJU Int 2020;125: 197–205.
[Crossref] [Google Scholar] [PubMed].
- Meeks JJ, Bellmunt J, Bochner BH, Clarke NW, Daneshmand S, Galsky MD, et al. A systematic review of neoadjuvant and adjuvant chemotherapy for muscle-invasive bladder cancer. Eur Urol 2012;62: 523–533.
[Crossref] [Google Scholar] [PubMed].
- Yu SS, Ballas LK, Skinner EC, Dorff TB, Sadeghi S, Quinn DI. Immunotherapy in urothelial cancer, part 2: Adjuvant, neoadjuvant, and adjunctive treatment. Clin Adv Hematol Oncol 2017;15: 543-551.
[Crossref] [Google Scholar] [PubMed].
- Rhea LP, Aragon-Ching JB. Advances and controversies with checkpoint inhibitors in bladder cancer. Clin Med Insights Oncol 2021;28: 15-63.
[Crossref] [Google Scholar] [PubMed].
- Vidotto T, Nersesian S, Graham C, Siemens DR, Koti M. DNA damage repair gene mutations and their association with tumor immune regulatory gene expression in muscle invasive bladder cancer subtypes. J Immunother Cancer 2019;7: 148.
[Crossref] [Google Scholar] [PubMed].
- Barone B, Calogero A, Scafuri L, Ferro M, Lucarelli G, Di Zazzo E, et al. Immune checkpoint inhibitors as a neoadju-vant/adjuvant treatment of muscle-invasive bladder cancer: A systematic review. Cancers (Basel) 2022;14: 2545.
[Crossref] [Google Scholar] [PubMed].
- Basile G, Bandini M, Gibb EA, Ross JS, Raggi D, Marandino L, et al. Neoadjuvant pembrolizumab and radical cystectomy in patients with muscle-invasive urothelial bladder cancer: 3-Year median follow-up update of PURE-01 trial. Clin Cancer Res 2022.
[Crossref] [Google Scholar].
- Mukherji D, Jabbour M, Saroufim M, Temraz S, Nasr R, Shamseddine A, et al. Programmed death-ligand 1 expression in muscle-invasive bladder cancer cystectomy specimens and lymph node metastasis. Clin Genitourin Cancer 2016;14: 183-187.
[Crossref] [Google Scholar] [PubMed].
- Rhea LP, Mendez-Marti S, Kim D, Aragon-Ching JB. Role of immunotherapy in bladder cancer. Cancer Treat Res Commun 2021;26: 100296.
[Crossref] [Google Scholar] [PubMed].
- Hoffmann LG, Sarian LO, Vassallo J, de Paiva Silva GR, Ramalho SOB, Ferracini AC, et al. Evaluation of PD-L1 and tumor infiltrating lymphocytes in paired pretreatment biopsies and post neoadjuvant chemotherapy surgical specimens of breast carcinoma. Sci Rep 2021;11: 22478.
[Crossref] [Google Scholar] [PubMed].
Copyright: © 2022 The Authors. This is an open access article under the terms of the Creative Commons Attribution NonCommercial ShareAlike 4.0 (https://creativecommons.org/licenses/by-nc-sa/4.0/). This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.