Case Report - Journal of Interdisciplinary Histopathology (2022)
Paratesticular Malignant Ectomesenchymoma: An Unrecognized Entity Case Report
Saulo Mendoza-Ramírez1,2*, Abril Trejo‐Caballero1, Marco Antonio Olvera‐Olvera3 and Mario Murguia‐Perez3*2Department of Surgical Pathology, ABC Observatory Medical Center, Mexico City, Mexico
3Department of Surgical Pathology, UMAE Specialty Hospital N° 1, National Medical Center Bajío, Mexican Institute of Social Security, León, Guanajuato, Mexico
Saulo Mendoza-Ramírez, Department of Surgical Pathology, Hospital General de México, Mexico city, Mexico, Email: dr.saulomr.oncopato@gmail.com
Mario Murguia‐Perez, Department of Surgical Pathology, UMAE Specialty Hospital N° 1, National Medical Center Bajío, Mexican Institute of Social Security, León, Guanajuato, Mexico, Email: drmariopatologia@gmail.com
Received: 23-Sep-2022, Manuscript No. EJMJIH-22-76277; Editor assigned: 26-Sep-2022, Pre QC No. EJMJIH-22-76277 (PQ); Reviewed: 10-Oct-2022, QC No. EJMJIH-22-76277; Revised: 17-Oct-2022, Manuscript No. EJMJIH-22-76277 (R); Published: 25-Oct-2022
Abstract
Malignant ectomesenchymoma is the malignant neoplasm that derives from this tissue; it is characterized by presenting differentiation of neuro-ectodermal and mesenchymal components. The mesenchymal component most of the time is rhabdomyosarcoma. We present the case of an 18-year-old man with a right paratesticular tumor and diffuse pulmonary nodules with probable metastatic activity. The initial diagnosis was a right paratesticular sarcoma with rhabdoid differentiation. By expanding the immunohistochemistry panel, the diagnosis of malignant ectomesenchymoma was confirmed. This tumor is rare and rapidly progressive; few cases have been described in the medical literature.
Keywords
Malignant ectomesenchymoma; rhabdomyosarcoma; paratesticular tumor
Introduction
The word ectomesenchyme refers to mesenchymal cells that are derived from the neural crest [1]. The term “ectomesenchyme” was proposed in 1950 by Hörstadius, to indicate the property of neural crest cells to give rise to many and varied mesenchymal and neuroectodermal elements. Malignant Ectomesenchymoma (MEM) which is derived from the remnants of migratory neural crest cells (ectomesenchyme); it is a rare and rapidly progressive tumor, composed of neuroectodermal and mesenchymal neoplastic elements. It occurs mainly in children and adolescents, originating in the perineal area (29%), abdomen (22%), head and neck (16%), brain (13%), extremities, and soft tissues (9%) [2-9]. The neuroectodermal component can be highly variable, from groups of ganglion cells to immature primitive neuronal elements only identified by immunohistochemical reactions. The mesenchymal component is generally rhabdomyosarcoma with a predominantly embryonal subtype; however, pleomorphic sarcoma, undifferentiated sarcoma, chondrosarcoma, liposarcoma, and gliosarcoma have been reported [3]. Due to its extreme rarity and heterogeneous composition, it is difficult to determine the best treatment option, prognostic factors and make general statements about its results. Surgery is the mainstay of treatment, but the role of adjuvant radiation and chemotherapy is still unclear [6-7]. Although quite limited, most of the existing data on patients with MEM, including cytogenetic abnormalities, clinical behavior, and therapeutic response, show overlap with features of rhabdomyosarcoma, suggesting that MEM might represent a variant of embryonal rhabdomyosarcoma. Contradictory to this, evidence from a genomic study on the so-called “intracranial MEM” revealed an expression profile closer to that of the malignant tumor of the peripheral nerve sheath [8].
According to the World Health Organization (WHO) classification, ectomesenchymoma belonged to the group of peripheral sheath tumors; however, this categorization was incorporated again and verified by a genetic profile study that suggests a rhabdomyosarcomatous differentiation [4], according to the 5th edition of the WHO in the classification of soft tissue and bone tumors, tumors categorized as ectomesenchymomas are considered skeletal muscle tumors [8]. Until 2015, only eight cases of scrotal MEM had been reported [2]. We present the case of a patient with a right paratesticular MEM of two months of evolution, which would represent case number 9 reported to date.
Case Presentation
An 18-year-old man, with no relevant history, began his condition two months prior to his hospital admission, presenting severe right testicular pain, increased volume, and a 4 kg weight loss, for which he went for medical evaluation. An ultrasound was performed in which a tumor lesion dependent on the right testicle was identified. Subsequently, an abdominopelvic computed tomography was performed, and a tumor was identified in the right testicle, as well as pulmonary nodules and retroperitoneal adenopathies, suggesting probable metastatic activity. Right radical orchiectomy was performed.
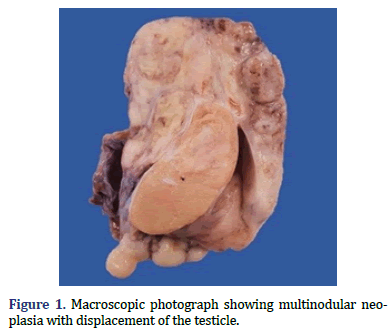
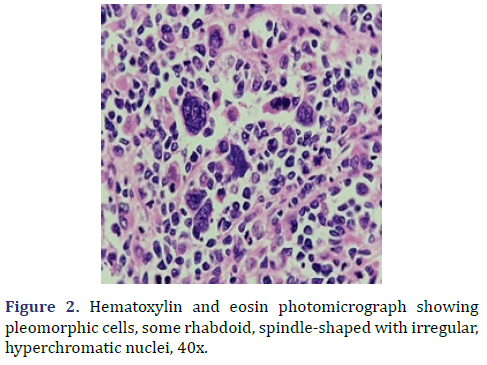
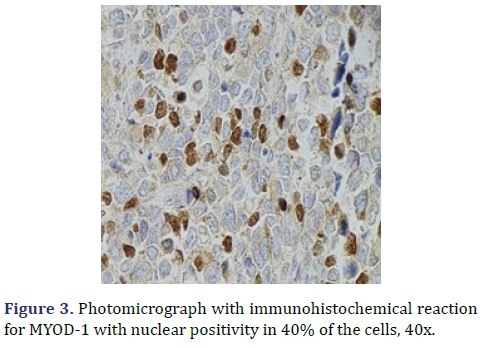
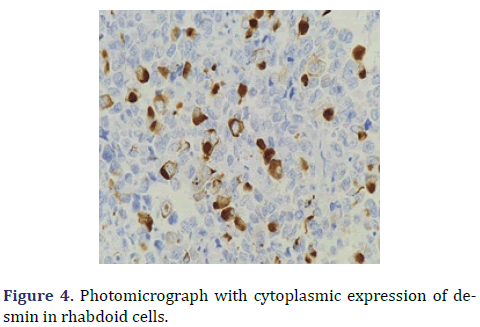
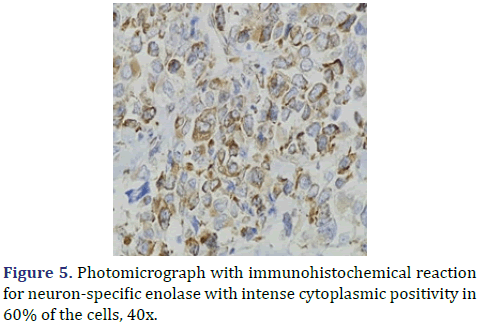
In the surgical pathology service, a surgical piece corresponding to the right testicle was received; a paratesticular, multinodular, heterogeneous tumor with areas of myxoid appearance and areas of necrosis was identified (Figure 1). Histologically, an encapsulated, multinodular lesion with fibrous septa was observed, with a growth pattern in nests made up of spindle cells, epithelioid cells with abundant eosinophilic cytoplasm with an eccentric nucleus and prominent nucleolus, multinucleated cells with hyperchromasia, and small, round cells with a hyperchromatic nucleus with marked pleomorphism (Figure 2). Initially, the immunohistochemical reactions led to the initial diagnosis of paratesticular rhabdomyosarcoma, however, the positivity in only 50% of the tumor cells for Myogenetic differentiation 1 MyoD-1 (Figure 3) and myogenin, led to the expansion of the panel, showing positivity for desmin in cells with rhabdoid differentiation (Figure 4), spindle cells positive for S100, chromogranin (focal) and neuron-specific enolase (Figure 5), confirming the presence of the neuroectodermal and mesenchymal component (rhabdomyosarcoma); Likewise, it presented a proliferation index of 50% (Ki-67). With the above, it was concluded as MEM.
Discussion
The term “ectomesenchyme” was proposed in 1950 by Hörstadius, to indicate the property of neural crest cells to give rise to many and varied mesenchymal and neuroectodermal elements; they can progressively diversify through the interaction of intrinsic and extrinsic influences of the local microenvironment [10]. They are characterized by a fusion of neuroectodermal and mesenchymal components [2-7]. They are composed of well differentiated or neuroblastic cells, Primitive NeuroEctodermal Tumors (PNET), and one or more malignant mesenchymal elements, usually of the rhabdomyosarcoma type. MEMs are extremely rare tumors; around 64 cases have been described. They occur mainly in children and adolescents and can arise in various sites, but most have been reported in the perineal/pelvic area, followed by head and neck, intracranial, extremities, intra-abdominal, and retroperitoneal. These tumors seem to have a slight male predominance (with a male-to-female ratio of 1:4) and are more commonly present in the first decade of life (82%) [3-8]. The essential criteria for its diagnosis are morphology composed of areas similar to an embryonal rhabdomyosarcoma interspersed with neuroblastic elements (neurons, ganglioneuroma, ganglioneuroblastoma, or neuroblastoma). MEM is often confused with rhabdomyosarcoma due to the fact that its neural components are easily overlooked.
The neuroectodermal component can be highly variable, from groups of ganglion cells to immature primitive neuronal elements only identified by immunohistochemical staining. The mesenchymal component is generally rhabdomyosarcoma with a predominantly embryonal subtype; however, pleomorphic sarcoma, undifferentiated sarcoma, chondrosarcoma, liposarcoma, and gliosarcoma have been reported [3-7]. General macroscopic features include a multilobulated appearance and thin encapsulation, with extensive areas of hemorrhage and necrosis. The size of the tumor varies from 3 to 18 cm, with the majority being 5 cm at the time of diagnosis [9].
Since MEM is a biphasic tumor with a variable percentage regarding the differentiation of its components, differential diagnoses range from well-differentiated sarcomas to poorly differentiated mesenchymal sarcomas or neuroectodermal tumors; tumors such as embryonal rhabdomyosarcoma, pleomorphic sarcoma, chondrosarcoma, undifferentiated sarcoma, ganglioneuroma, neuroblastoma, primitive neuroectodermal tumor and malignant peripheral nerve sheath tumor [3-5]. Immunohistochemical reactions are essential to highlight the double component of this neoplasm. The presence of mesenchymal and neural elements with immunohistochemical reactivity for myogenin and/or desmin, CD56, PGP9.5, synaptophysin, chromogranin, and tyrosine hydroxylase 3-9 should be demonstrated. We recommend suspecting the entity when the immunohistochemical markers do not have a diffuse or conclusive positivity, the manifestations are weak and, above all, when the expression is heterogeneous or in patches, this moment is when it becomes necessary to demonstrate the immunophenotype of the entire tumor. Apart from the clinicopathological characteristics, the MEM shows a high frequency (86%) with HRAS mutations; other rare mutations detected involve the PTPRD and FBXW78 genes.
The correct diagnosis of MEM is clinically relevant, to its proper approach and selection of treatment. Due to its extreme rarity and heterogeneous composition, it is difficult to determine the best treatment option. Currently within the prognostic factors are an age <3 years, size <10 cm, and a superficial location. Surgery is the most adequate treatment, but the role of adjuvant radiation and chemotherapy is still uncertain [6,7]. The main predictive characteristic of a good prognosis in a MEM is the possibility of being resected; patients who have succumbed to the disease have had unresectable primary tumors or metastasis at the time of diagnosis [5].
Conclusion
MEM is rare tumors that must be considered due to the clinical implications observed in the few documented cases. Immunohistochemistry is essential for diagnosis based on the morphology observed with hematoxylin/eosin staining. Above all, we recommend expanding the battery of antibodies when the markers do not have a diffuse expression of a cell line, the entity must be suspected when the neural or skeletal muscle markers are positive in variable percentages of the tumor. Treatment is based on rhabdomyosarcomas as primitive neuroblastic elements do not appear to have an impact on treatment.
Orcid
Mario Murguia-Perez https://orcid.org/0000-0003- 4260-389X
References
- Millán Y, Martínez Í, Moram E. Ectomesenquimoma Maligno: A Propósito de un Caso. Revista Venezolana de Oncología 2009;2: 109-112.
- Kao W-T, Chiang Y-T, Tzou K-Y. An Adult Paratesticular Malignant Ectomesenchymoma With Post-operative Flare-up of Lung Metastasis. Urol Case Rep 2015;3: 164-166.
[Crossref] [Google Scholar] [PubMed]
- Nael K, Siaghani P, Wu W, Nael K, Shane L, Romansky G. Metastatic Malignant Ectomesenchymoma Initially Presenting as a Pelvic Mass: Report of a Case and Review of Literature. Case Rep Pediatr (2014);4: 1-9.
[Crossref] [Google Scholar] [PubMed]
- Howley S, Stack D, Morris T, McDermott M, Capra M, Betts D et al. Ectomesenchymoma with t(1;12) (p32;p13) evolving from embryonal rhabdomyosarcoma shows no rearrangement of ETV6. Hum Pathol 2012;43: 299-302.
[Crossref] [Google Scholar] [PubMed]
- E. Yohe M, Girard ED, Balarezo FS, Browna RT, Wu AC, Finck CM et al. A novel case of pediatric thoracic malignant ectomesenchymoma in an infant. Journal of Pediatric Surgery Case Reports 2013;1: 20-22.
- Dantonello TM, Leuschner I, Vokuhl C, Gfroerer S, Schuck A, Kube S et al. Malignant Ectomesenchymoma in Children and Adolescents: Report from the Cooperative Weichteilsarkom Studiengruppe (CWS). Pediatr Blood Cancer 2013;60: 224-229.
[Crossref] [Google Scholar] [PubMed]
- Vandenheuvel K, Carpentieri D, Chen J, Fung K, Parham D. Ectomesenchymoma with embryonal rhabdomyosarcoma and ganglioneuroma, arising in association with benign triton tumor of the tongue. Pediatr Dev Pathol 2014;17: 226-230.
[Crossref] [Google Scholar] [PubMed]
- Huang S-C, Alaggio R, Sung Y-S, Chen C-L, Zhang L, Kao Y-C et al. Frequent HRAS Mutations in Malignant Ectomesenchymoma: Overlapping Genetic Abnormalities With Embryonal Rhabdomyosarcoma. Am J Surg Pathol 2016;40: 1-2.
[Crossref] [Google Scholar] [PubMed]
- Boue DR, Parham DM, Webber B, Crist WM, Qualman SJ. Clinicopathologic Study of Ectomesenchymomas from Intergroup Rhabdomyosarcoma Study Groups III and IV. Pediatr Dev Pathol 2000;3: 290-300.
[Crossref] [Google Scholar] [PubMed]
- Freitas AB, Aguiar PH, Miura FK, Yasuda A, Soglia J, Soglia F et al. Malignant ectomesenchymoma: Case report and review of the literature. Pediatr Neurosurg1999;30: 320-330.
[Crossref] [Google Scholar] [PubMed]
Copyright: © 2022 The Authors. This is an open access article under the terms of the Creative Commons Attribution NonCommercial ShareAlike 4.0 (https://creativecommons.org/licenses/by-nc-sa/4.0/). This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.