Case Report - Journal of Interdisciplinary Histopathology (2022)
Vaginal Mucosa Melanoma With Giant Cell Phenotype: A Case Report
Saulo Mendoza-Ramírez1,2*, Saray Betsabe Santos Torres1, Leonora Chávez Mercado1, Lourdes Lucía Morales-Jáuregui3, Marco Antonio Olvera-Olvera3 and Mario Murguia-Perez3*2Department of Surgical Pathology, ABC Observatory Medical Center, Mexico, Mexico
3Department of Surgical Pathology, Mexican Institute of Social Security, León, Guanajuato, Mexico
Saulo Mendoza-Ramírez, Department of Surgical Pathology, ABC Observatory Medical Center, Mexico, Mexico,
Mario Murguia-Perez, Department of Surgical Pathology, Mexican Institute of Social Security, León, Guanajuato, Mexico, Email: drmariopatologia@gmail.com
Received: 22-Sep-2022, Manuscript No. EJMJIH-22-76882; Editor assigned: 26-Sep-2022, Pre QC No. EJMJIH-22-76882 (PQ); Reviewed: 11-Oct-2022, QC No. EJMJIH-22-76882; Revised: 18-Oct-2022, Manuscript No. EJMJIH-22-76882 (R); Published: 26-Oct-2022
Abstract
Melanomas are malignant tumors that arise from pigmented cells, melanocytes. In the female genital tract, they represent 18% of all mucosal melanomas. The first vaginal melanoma was described in 1887, and approximately 500 cases have recently been documented in the literature. Currently, vaginal melanoma represents <5.5% of all vaginal neoplasms and 0.4 to 0.8% of all melanomas in women. We present the case of a 60 year-old woman with transvaginal bleeding and dysuria. Colposcopy reported< an exophytic lesion located on the anterior vaginal wall, pigmented and bleeding upon contact. The clinical diagnosis was cervical carcinoma, a biopsy was taken and sent to the Surgical Pathology service where the histopathological diagnosis of melanoma with multinucleated giant cell phenotype of the vaginal mucosa was concluded, it also presented pigmentation and osteoid stromal metaplasia.
Keywords
Melanoma of the vaginal mucosa; Multinucleated giant cells; Histological variants; Immunohistochemistry; BRAF
Case Presentation
A 60 year-old woman with a reported gynecological- obstetric history: 2 gestations of those 2 normal deliveries, 0 abortions, and menarche at 13 years and menopause at 50 years, mammography and cervical cytology never performed. Her condition began five months earlier with transvaginal bleeding and dysuria. Colposcopy showed an exophytic lesion located on the anterior vaginal wall, pigmented and bleeding on contact; with a partial vision of the exocervix and endocervix, erythematous, and with mucopurulent discharge.
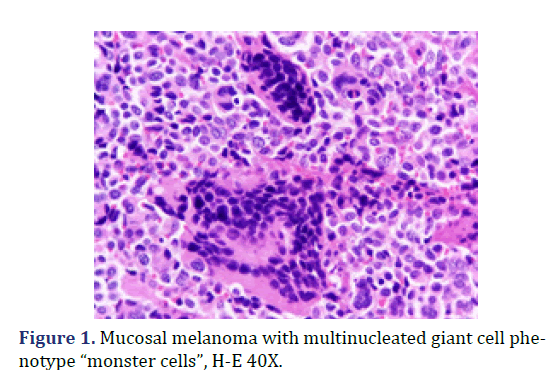
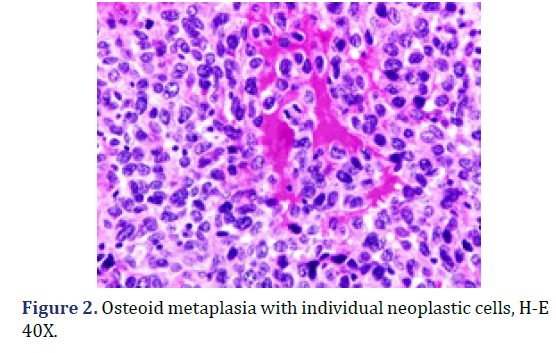
A biopsy was taken and sent to Department of Surgical Pathology, Hospital General de México “Dr. Eduardo Liceaga”, for its diagnostic evaluation. In the service, a bottle with formaldehyde was received, referred with the clinical diagnosis of “cervical cancer”; with multiple fragments of irregular shape and surface, with a combined measurement of 3.0 cm x 1.5 cm x 1.0 cm, of friable consistency, reddish brown coloring, mostly covered with hemorrhagic remains. Microscopic evaluation revealed non-keratinized, ulcerated flat stratified residual epithelium and an epithelial-type lesion, 40% formed by multinucleated giant cells of the osteoclast type (Figure 1), with moderate and eosinophilic cytoplasm, pleomorphic nuclei, and dense chromatin; in a background of atypical cells, some binucleated, with abundant extravasated erythrocytes and few inflammatory cells. Two foci of osteoid metaplasia were identified, surrounding the neoplastic cells (Figure 2), as well as a few neoplastic cells with cytoplasmic melanin pigment.
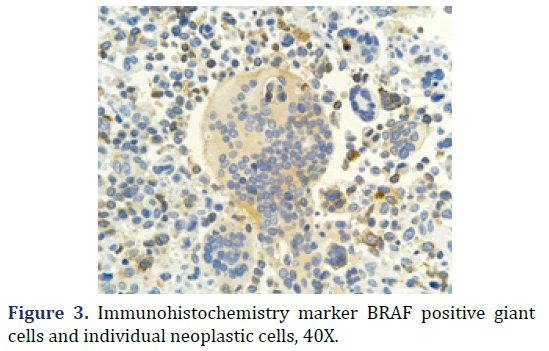
The histopathological diagnosis was malignant melanoma of the vaginal mucosa. Immunohistochemistry (IHC) markers were performed for Microphthalmia Transcription Factor (MITF), HMB-45, PS100, and Melan-A, which were positive with expression in giant cells and individual neoplastic cells; confirming the diagnosis issued (Figure 3). BRAF was included in the panel as a possible therapeutic route targeting the specific mutation, which showed positivity with mild intensity in multinucleated giant cells. The case was consulted with one of the authors at the UMAE Hospital de Especialidades, Centro Médico Nacional Bajío, IMSS, agreeing with the final diagnosis.
The patient did not continue the treatment and follow-up issued by the Oncology Department of the same hospital; the course and outcome of the patient are unknown.
Results and Discussion
The first vaginal melanoma was described in 1887 and approximately 500 cases have recently been reported in the literature [3]. We describe an entity that, due to its location and morphology, is infrequent. The cases reported in the literature of melanomas with a giant cell phenotype were all cutaneous and mostly in the head and neck region. Macroscopic pigment is a feature of melanoma and must be differentiated from other pigmented melanoses, such as melanosis, lentigo, and nevi, particularly dysplastic nevi [3]. Diagnosis of primary mucosal melanoma, especially in sites where it rarely arises, is of crucial importance to exclude the possibility of a metastatic lesion [2]. In general, melanoma can present a wide range of architectural patterns, cytological characteristics, and stromal changes; sometimes in combination with these, which makes diagnosis more complex and challenging when pigmented areas are absent.
Unlike other solid malignancies, histologic subtypes of primary melanoma are not frequently reported in pathology reports.
The SEER cut-off analysis shows that the histological subtype is an independent predictor of survival in melanoma [13], contrary to the location and adjacent invasion.
The morphological examination continues to be the gold standard for diagnosis, due to the wide morphology that it presents, studies with IHC biomarkers are often required for diagnostic confirmation [11]. Among all IHC markers, S100 and SOX10 are the most sensitive to melanocytic lesions, although they lack specificity. Melanoma-recognized T-cell augmentation (MART-1), MITF, and HMB-45 are frequently used because they have higher specificity and reasonable sensitivity in differentiating most conventional melanomas from histological non-melanocytic mimics [2].
The 8° edition of the American Joint Committee on Cancer (AJCC) included the TNM system for the staging of mucosal melanoma of the head and neck, but the establishment of appropriate systems for the other locations is necessary [3]; historical adaptations such as the Ballantyne system, where it is classified as stage I: localized disease, stage II: regional lymph node involvement, and stage III: distant metastatic disease; serve for the non-exclusive staging of an organ [14]. The future existence of a universal staging system for mucosal melanomas could provide adequate classification, treatment planning, and prognosis; also allow meaningful comparison of results from various institutions to define the best treatment options [3].
Conclusion
To date there is no effective systemic therapy; most studies focus on genetics and underlying molecular events. Therefore, mutation analysis of proto-oncogene tyrosine kinase (c-KIT), Neuroblastoma Rat Sarcoma (NRAS), and proto-oncogene B-Raf serine/threonine kinase (BRAF) are recommended at diagnosis initial and recurrence in all patients with female genital melanoma. In our case, BRAF expression made the patient a candidate for one of the specific drugs such as Vemurafenib (Zelboraf), Dabrafenib (Tafinlar), and Encorafenib (Braftovi); Unfortunately, with loss of clinical follow-up due to abandonment of treatment, the outcome of this case is unknown.
Orcid
Mario Murguia-Perez https://orcid.org/0000-0003-4260-389X
References
- Yde SS, Sjoegren P, Heje M, Stolle LB. Mucosal Melanoma: A Literature Review. Curr Oncol Rep 2018;20: 28.
[Crossref] [Google Scholar] [PubMed].
- Mihajlovic M, Vlajkovic S, Jovanovic P, Stefanovic V. Primary mucosal melanomas: A comprehensive review. Int J Clin Exp Pathol 2012;5: 739–753.
[Google Scholar] [PubMed].
- Gadducci A, Carinelli S, Guerrieri ME, Aletti GD. Melanoma of the lower genital tract: Prognostic factors and treatment modalities. Gynecol Oncol 2018;150: 180–189.
[Crossref] [Google Scholar] [PubMed].
- Trone JC, Guy JB, Mery B, Escure JL, Lahmar R, Moncharmont C, et al. Mélanomes du tractus génital féminin: État des lieux. Bull Cancer 2014;101: 102–106.
[Crossref] [Google Scholar] [PubMed].
- Wohlmuth C, Wohlmuth-Wieser I, May T, Vicus D, Gien LT, Laframboise S. Malignant Melanoma of the Vulva and Vagina: A US Population-Based Study of 1863 Patients. Am J Clin Dermatol 2020;21: 285–295.
[Crossref] [Google Scholar].
- Spencer KR, Mehnert JM. Mucosal Melanoma: Epidemiology, Biology and Treatment. Cancer Treat Res 2016; 167: 295-320.
[Crossref] [Google Scholar] [PubMed].
- Pandey G, Dave P, Patel S, Patel B, Arora R, Parekh C, et al. Female genital tract melanoma: Analysis from a regional cancer institute. Turk Jinekoloji ve Obstet Dern Derg 2020;17: 46–51.
[Crossref] [Google Scholar] [PubMed].
- Dumaz N, Jouenne F, Delyon J, Mourah S, Bensussan A, Lebbé C. Atypical BRAF and NRAS mutations in mucosal melanoma. Cancers (Basel) 2019;11: 1133.
[Crossref] [Google Scholar] [PubMed].
- Rongioletti F, Smoller BR. Unusual histological variants of cutaneous malignant melanoma with some clinical and possible prognostic correlations. J Cutan Pathol 2005;32: 589–603.
[Crossref] [Google Scholar] [PubMed].
- Cota C, Saggini A, Lora V, Kutzner H, Rütten A, Sangüeza O, et al. Uncommon Histopathological Variants of Malignant Melanoma: Part 1. Am J Dermatopathol 2019;41: 243–263.
[Crossref] [Google Scholar] [PubMed].
- Hirsch MS, Watkins J. A Comprehensive Review of Biomarker Use in the Gynecologic Tract Including Differential Diagnoses and Diagnostic Pitfalls. Adv Anat Pathol 2019;27: 164–192.
[Crossref] [Google Scholar] [PubMed].
- Zhu H, Dong D, Li F, Liu D, Wang L, Fu J, et al. Clinicopathologic features and prognostic factors in patients with non-cutaneous malignant melanoma: A single-center retrospective study of 71 cases. Int J Dermatol 2015;54: 1390–1395.
[Crossref] [Google Scholar] [PubMed].
- Lattanzi M, Lee Y, Simpson D, Moran U, Darvishian F, Kim RH, et al. Primary melanoma histologic subtype: Impact on survival and response to therapy. J Natl Cancer Inst 2019;111: 186–188.
[Crossref] [Google Scholar] [PubMed].
- Sánchez RB, Bustos BU, Mira MN, Estrada RB. Actualización en melanoma mucoso. Actas Dermosifiliogr 2015;106: 96–103.
[Crossref] [Google Scholar] [PubMed].
- Sanchez A, Rodríguez D, Allard CB, Bechis SK, Sullivan RJ, Boeke CE, et al. Primary genitourinary melanoma: Epidemiology and disease-specific survival in a large population-based cohort. Urol Oncol 2016;34: 166.e7-166.e14.
[Crossref] [Google Scholar] [PubMed].
Copyright: © 2022 The Authors. This is an open access article under the terms of the Creative Commons Attribution NonCommercial ShareAlike 4.0< (https://creativecommons.org/licenses/by-nc-sa/4.0/). This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.